Little Evidence of Antagonistic Selection in the Evolutionary Strata of Fungal Mating-Type Chromosomes (Microbotryum lychnidis-dioicae)
- PMID: 31015196
- PMCID: PMC6553529
- DOI: 10.1534/g3.119.400242
Little Evidence of Antagonistic Selection in the Evolutionary Strata of Fungal Mating-Type Chromosomes (Microbotryum lychnidis-dioicae)
Abstract
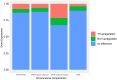
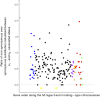
Recombination suppression on sex chromosomes often extends in a stepwise manner, generating evolutionary strata of differentiation between sex chromosomes. Sexual antagonism is a widely accepted explanation for evolutionary strata, postulating that sets of genes beneficial in only one sex are successively linked to the sex-determining locus. The anther-smut fungus Microbotryum lychnidis-dioicae has mating-type chromosomes with evolutionary strata, only some of which link mating-type genes. Male and female roles are non-existent in this fungus, but mating-type antagonistic selection can also generate evolutionary strata, although the life cycle of the fungus suggests it should be restricted to few traits. Here, we tested the hypothesis that mating-type antagonism may have triggered recombination suppression beyond mating-type genes in M. lychnidis-dioicae by searching for footprints of antagonistic selection in evolutionary strata not linking mating-type loci. We found that these evolutionary strata (i) were not enriched in genes upregulated in the haploid phase, where cells are of alternative mating types, (ii) carried no gene differentially expressed between mating types, and (iii) carried no genes displaying footprints of specialization in terms of protein sequences (dN/dS) between mating types after recommended filtering. Without filtering, eleven genes showed signs of positive selection in the strata not linking mating-type genes, which constituted an enrichment compared to autosomes, but their functions were not obviously involved in antagonistic selection. Thus, we found no strong evidence that antagonistic selection has contributed to extending recombination suppression beyond mating-type genes. Alternative hypotheses should therefore be explored to improve our understanding of the sex-related chromosome evolution.
Keywords: Genetics of Sex; antagonistic selection; evolutionary strata; expression; fungi; haploid selection; mating-type chromosomes; sex chromosomes; sexual antagonism.
Copyright © 2019 Bazzicalupo et al.
Figures




Similar articles
-
Differential Gene Expression between Fungal Mating Types Is Associated with Sequence Degeneration.Genome Biol Evol. 2020 Apr 1;12(4):243-258. doi: 10.1093/gbe/evaa028. Genome Biol Evol. 2020. PMID: 32058544 Free PMC article.
-
Recombination suppression and evolutionary strata around mating-type loci in fungi: documenting patterns and understanding evolutionary and mechanistic causes.New Phytol. 2021 Mar;229(5):2470-2491. doi: 10.1111/nph.17039. Epub 2020 Dec 1. New Phytol. 2021. PMID: 33113229 Free PMC article. Review.
-
Chaos of Rearrangements in the Mating-Type Chromosomes of the Anther-Smut Fungus Microbotryum lychnidis-dioicae.Genetics. 2015 Aug;200(4):1275-84. doi: 10.1534/genetics.115.177709. Epub 2015 Jun 3. Genetics. 2015. PMID: 26044594 Free PMC article.
-
Deciphering evolutionary strata on plant sex chromosomes and fungal mating-type chromosomes through compositional segmentation.Plant Mol Biol. 2016 Mar;90(4-5):359-73. doi: 10.1007/s11103-015-0422-y. Epub 2015 Dec 22. Plant Mol Biol. 2016. PMID: 26694866
-
Fungal Sex: The Basidiomycota.Microbiol Spectr. 2017 Jun;5(3):10.1128/microbiolspec.funk-0046-2016. doi: 10.1128/microbiolspec.FUNK-0046-2016. Microbiol Spectr. 2017. PMID: 28597825 Free PMC article. Review.
Cited by
-
Differential Gene Expression between Fungal Mating Types Is Associated with Sequence Degeneration.Genome Biol Evol. 2020 Apr 1;12(4):243-258. doi: 10.1093/gbe/evaa028. Genome Biol Evol. 2020. PMID: 32058544 Free PMC article.
-
Onset and stepwise extensions of recombination suppression are common in mating-type chromosomes of Microbotryum anther-smut fungi.J Evol Biol. 2022 Dec;35(12):1619-1634. doi: 10.1111/jeb.13991. Epub 2022 Mar 10. J Evol Biol. 2022. PMID: 35271741 Free PMC article.
-
An Inversion Polymorphism Under Balancing Selection, Involving Giant Mobile Elements, in an Invasive Fungal Pathogen.Mol Biol Evol. 2025 Feb 3;42(2):msaf026. doi: 10.1093/molbev/msaf026. Mol Biol Evol. 2025. PMID: 39907064 Free PMC article.
-
Recombination suppression and evolutionary strata around mating-type loci in fungi: documenting patterns and understanding evolutionary and mechanistic causes.New Phytol. 2021 Mar;229(5):2470-2491. doi: 10.1111/nph.17039. Epub 2020 Dec 1. New Phytol. 2021. PMID: 33113229 Free PMC article. Review.
-
Sheltering of deleterious mutations explains the stepwise extension of recombination suppression on sex chromosomes and other supergenes.PLoS Biol. 2022 Jul 19;20(7):e3001698. doi: 10.1371/journal.pbio.3001698. eCollection 2022 Jul. PLoS Biol. 2022. Retraction in: PLoS Biol. 2025 Mar 27;23(3):e3003106. doi: 10.1371/journal.pbio.3003106. PMID: 35853091 Free PMC article. Retracted.
References
-
- Alexander H. M., 1989. An experimental field-study of anther-smut disease of Silene alba caused by Ustilago violacea- Genotypic variation and disease incidence. Evolution 43: 835–847. - PubMed
-
- Alexander H. M., 1990. Epidemiology of anther-smut infection of Silene alba caused by Ustilabo violacea - patterns of spore deposition and disease incidence. J. Ecol. 78: 166–179. 10.2307/2261043 - DOI
-
- Alexander H. M., Antonovics J., 1995. Spread of anther-smut disease (Ustilago violacea) and character correlations in a genetically variable population of Silene alba. J. Ecol. 83: 783–794. 10.2307/2261415 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
