StarD5: an ER stress protein regulates plasma membrane and intracellular cholesterol homeostasis
- PMID: 31015253
- PMCID: PMC6547630
- DOI: 10.1194/jlr.M091967
StarD5: an ER stress protein regulates plasma membrane and intracellular cholesterol homeostasis
Abstract
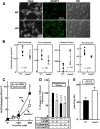
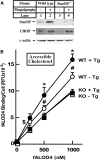
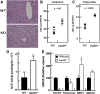
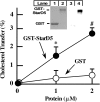
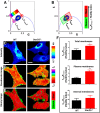
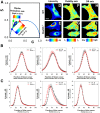
How plasma membrane (PM) cholesterol is controlled is poorly understood. Ablation of the gene encoding the ER stress steroidogenic acute regulatory-related lipid transfer domain (StarD)5 leads to a decrease in PM cholesterol content, a decrease in cholesterol efflux, and an increase in intracellular neutral lipid accumulation in macrophages, the major cell type that expresses StarD5. ER stress increases StarD5 expression in mouse hepatocytes, which results in an increase in accessible PM cholesterol in WT but not in StarD5-/- hepatocytes. StarD5-/- mice store higher levels of cholesterol and triglycerides, which leads to altered expression of cholesterol-regulated genes. In vitro, a recombinant GST-StarD5 protein transfers cholesterol between synthetic liposomes. StarD5 overexpression leads to a marked increase in PM cholesterol. Phasor analysis of 6-dodecanoyl-2-dimethylaminonaphthalene fluorescence lifetime imaging microscopy data revealed an increase in PM fluidity in StarD5-/- macrophages. Taken together, these studies show that StarD5 is a stress-responsive protein that regulates PM cholesterol and intracellular cholesterol homeostasis.
Keywords: Niemann-Pick C; cholesterol trafficking; endoplasmic reticulum; fatty liver; fluorescence; macrophages; steroidogenic acute regulatory protein-related lipid transfer proteins; steroidogenic acute regulatory-related lipid transfer domain 5.
Figures

References
-
- Brown M. S., and Goldstein J. L.. 1986. A receptor-mediated pathway for cholesterol homeostasis. Science. 232: 34–47. - PubMed
-
- Jacobs N. L., Andemariam B., Underwood K. W., Panchalingam K., Sternberg D., Kielian M., and Liscum L.. 1997. Analysis of a Chinese hamster ovary cell mutant with defective mobilization of cholesterol from the plasma membrane to the endoplasmic reticulum. J. Lipid Res. 38: 1973–1987. - PubMed
-
- DeGrella R. F., and Simoni R. D.. 1982. Intracellular transport of cholesterol to the plasma membrane. J. Biol. Chem. 257: 14256–14262. - PubMed
-
- Simons K., and Ikonen E.. 2000. How cells handle cholesterol. Science. 290: 1721–1726. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

