Inflammasomes in neuroinflammatory and neurodegenerative diseases
- PMID: 31015277
- PMCID: PMC6554670
- DOI: 10.15252/emmm.201810248
Inflammasomes in neuroinflammatory and neurodegenerative diseases
Abstract
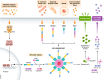
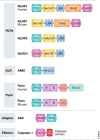
Neuroinflammation and neurodegeneration often result from the aberrant deposition of aggregated host proteins, including amyloid-β, α-synuclein, and prions, that can activate inflammasomes. Inflammasomes function as intracellular sensors of both microbial pathogens and foreign as well as host-derived danger signals. Upon activation, they induce an innate immune response by secreting the inflammatory cytokines interleukin (IL)-1β and IL-18, and additionally by inducing pyroptosis, a lytic cell death mode that releases additional inflammatory mediators. Microglia are the prominent innate immune cells in the brain for inflammasome activation. However, additional CNS-resident cell types including astrocytes and neurons, as well as infiltrating myeloid cells from the periphery, express and activate inflammasomes. In this review, we will discuss current understanding of the role of inflammasomes in common degenerative diseases of the brain and highlight inflammasome-targeted strategies that may potentially treat these diseases.
Keywords: disease; inflammasome; inflammation; microglia; neurodegeneration.
© 2019 The Authors. Published under the terms of the CC BY 4.0 license.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Inflammasomes as therapeutic targets in human diseases.Signal Transduct Target Ther. 2021 Jul 2;6(1):247. doi: 10.1038/s41392-021-00650-z. Signal Transduct Target Ther. 2021. PMID: 34210954 Free PMC article. Review.
-
NLRP3 Inflammasome Is Expressed and Functional in Mouse Brain Microglia but Not in Astrocytes.PLoS One. 2015 Jun 19;10(6):e0130624. doi: 10.1371/journal.pone.0130624. eCollection 2015. PLoS One. 2015. PMID: 26091541 Free PMC article.
-
Innate immune activation in neurodegenerative disease.Nat Rev Immunol. 2014 Jul;14(7):463-77. doi: 10.1038/nri3705. Nat Rev Immunol. 2014. PMID: 24962261 Review.
-
Inflammasomes in neurodegenerative diseases.Transl Neurodegener. 2024 Dec 23;13(1):65. doi: 10.1186/s40035-024-00459-0. Transl Neurodegener. 2024. PMID: 39710713 Free PMC article. Review.
-
Mechanisms of NLRP3 activation and pathology during neurodegeneration.Int J Biochem Cell Biol. 2022 Oct;151:106273. doi: 10.1016/j.biocel.2022.106273. Epub 2022 Aug 1. Int J Biochem Cell Biol. 2022. PMID: 35926782 Review.
Cited by
-
NLRP3 Deficiency Protects Against Intermittent Hypoxia-Induced Neuroinflammation and Mitochondrial ROS by Promoting the PINK1-Parkin Pathway of Mitophagy in a Murine Model of Sleep Apnea.Front Immunol. 2021 Feb 24;12:628168. doi: 10.3389/fimmu.2021.628168. eCollection 2021. Front Immunol. 2021. PMID: 33717152 Free PMC article.
-
Perfluoropentane-based oxygen-loaded nanodroplets reduce microglial activation through metabolic reprogramming.Neural Regen Res. 2025 Apr 1;20(4):1178-1191. doi: 10.4103/NRR.NRR-D-23-01299. Epub 2024 Apr 3. Neural Regen Res. 2025. PMID: 38989955 Free PMC article.
-
The extended autonomic system, dyshomeostasis, and COVID-19.Clin Auton Res. 2020 Aug;30(4):299-315. doi: 10.1007/s10286-020-00714-0. Epub 2020 Jul 22. Clin Auton Res. 2020. PMID: 32700055 Free PMC article. Review.
-
Therapeutic modulation of inflammasome pathways.Immunol Rev. 2020 Sep;297(1):123-138. doi: 10.1111/imr.12908. Epub 2020 Aug 7. Immunol Rev. 2020. PMID: 32770571 Free PMC article. Review.
-
Functional Crosstalk between CB and TRPV1 Receptors Protects Nigrostriatal Dopaminergic Neurons in the MPTP Model of Parkinson's Disease.J Immunol Res. 2020 Sep 28;2020:5093493. doi: 10.1155/2020/5093493. eCollection 2020. J Immunol Res. 2020. PMID: 33062722 Free PMC article.
References
-
- Allan SM, Tyrrell PJ, Rothwell NJ (2005) Interleukin‐1 and neuronal injury. Nat Rev Immunol 5: 629–640 - PubMed
-
- Baecher‐Allan C, Kaskow BJ, Weiner HL (2018) Multiple sclerosis: mechanisms and immunotherapy. Neuron 97: 742–768 - PubMed
-
- Baldwin AG, Brough D, Freeman S (2016) Inhibiting the inflammasome: a chemical perspective. J Med Chem 59: 1691–1710 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

