The Structural Basis for a Transition State That Regulates Pore Formation in a Bacterial Toxin
- PMID: 31015325
- PMCID: PMC6479001
- DOI: 10.1128/mBio.00538-19
The Structural Basis for a Transition State That Regulates Pore Formation in a Bacterial Toxin
Abstract
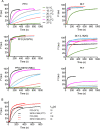
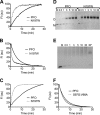
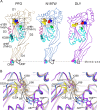
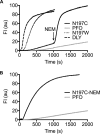
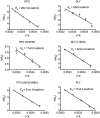
The cholesterol-dependent cytolysin (CDC) genes are present in bacterial species that span terrestrial, vertebrate, and invertebrate niches, which suggests that they have evolved to function under widely different environmental conditions. Using a combination of biophysical and crystallographic approaches, we reveal that the relative stability of an intramolecular interface in the archetype CDC perfringolysin O (PFO) plays a central role in regulating its pore-forming properties. The disruption of this interface allows the formation of the membrane spanning β-barrel pore in all CDCs. We show here that the relative strength of the stabilizing forces at this interface directly impacts the energy barrier posed by the transition state for pore formation, as reflected in the Arrhenius activation energy (Ea) for pore formation. This change directly impacts the kinetics and temperature dependence of pore formation. We further show that the interface structure in a CDC from a terrestrial species enables it to function efficiently across a wide range of temperatures by minimizing changes in the strength of the transition state barrier to pore formation. These studies establish a paradigm that CDCs, and possibly other β-barrel pore-forming proteins/toxins, can evolve significantly different pore-forming properties by altering the stability of this transitional interface, which impacts the kinetic parameters and temperature dependence of pore formation.IMPORTANCE The cholesterol-dependent cytolysins (CDCs) are the archetype for the superfamily of oligomeric pore-forming proteins that includes the membrane attack complex/perforin (MACPF) family of immune defense proteins and the stonefish venom toxins (SNTX). The CDC/MACPF/SNTX family exhibits a common protein fold, which forms a membrane-spanning β-barrel pore. We show that changing the relative stability of an extensive intramolecular interface within this fold, which is necessarily disrupted to form the large β-barrel pore, dramatically alters the kinetic and temperature-dependent properties of CDC pore formation. These studies show that the CDCs and other members of the CDC/MACPF/SNTX superfamily have the capacity to significantly alter their pore-forming properties to function under widely different environmental conditions encountered by these species.
Keywords: cold denaturation; complement; gasdermin; perforin; water network.
Copyright © 2019 Wade et al.
Figures

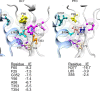
References
-
- Ellisdon AM, Reboul CF, Panjikar S, Huynh K, Oellig CA, Winter KL, Dunstone MA, Hodgson WC, Seymour J, Dearden PK, Tweten RK, Whisstock JC, McGowan S. 2015. Stonefish toxin defines an ancient branch of the perforin-like superfamily. Proc Natl Acad Sci U S A 112:15360–15365. doi:10.1073/pnas.1507622112. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

