Transcriptional Regulation of Voltage-Gated Sodium Channels Contributes to GM-CSF-Induced Pain
- PMID: 31015342
- PMCID: PMC6595947
- DOI: 10.1523/JNEUROSCI.2204-18.2019
Transcriptional Regulation of Voltage-Gated Sodium Channels Contributes to GM-CSF-Induced Pain
Abstract
Granulocyte-macrophage colony-stimulating factor (GM-CSF) induces the production of granulocyte and macrophage populations from the hematopoietic progenitor cells; it is one of the most common growth factors in the blood. GM-CSF is also involved in bone cancer pain development by regulating tumor-nerve interactions, remodeling of peripheral nerves, and sensitization of damage-sensing (nociceptive) nerves. However, the precise mechanism for GM-CSF-dependent pain is unclear. In this study, we found that GM-CSF is highly expressed in human malignant osteosarcoma. Female Sprague Dawley rats implanted with bone cancer cells develop mechanical and thermal hyperalgesia, but antagonizing GM-CSF in these animals significantly reduced such hypersensitivity. The voltage-gated Na+ channels Nav1.7, Nav1.8, and Nav1.9 were found to be selectively upregulated in rat DRG neurons treated with GM-CSF, which resulted in enhanced excitability. GM-CSF activated the Janus kinase 2 (Jak2)-signal transducer and activator of transcription protein 3 (Stat3) signaling pathway, which promoted the transcription of Nav1.7-1.9 in DRG neurons. Accordingly, targeted knocking down of either Nav1.7-1.9 or Jak2/Stat3 in DRG neurons in vivo alleviated the hyperalgesia in male Sprague Dawley rats. Our findings describe a novel bone cancer pain mechanism and provide a new insight into the physiological and pathological functions of GM-CSF.SIGNIFICANCE STATEMENT It has been reported that granulocyte-macrophage colony-stimulating factor (GM-CSF) plays a key role in bone cancer pain, yet the underlying mechanisms involved in the GM-CSF-mediated signaling pathway in nociceptors is not fully understood. Here, we showed that GM-CSF promotes bone cancer-associated pain by enhancing the excitability of DRG neurons via the Janus kinase 2 (Jak2)-signal transducer and activator of transcription protein 3 (Stat3)-mediated upregulation of expression of nociceptor-specific voltage-gated sodium channels. Our study provides a detailed understanding of the roles that sodium channels and the Jak2/Stat3 pathway play in the GM-CSF-mediated bone cancer pain; our data also highlight the therapeutic potential of targeting GM-CSF.
Keywords: DRG; GM-CSF; Jak2-Stat3; bone cancer pain; neural excitability; voltage-gated sodium channels.
Copyright © 2019 the authors.
Figures
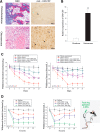


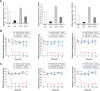

Similar articles
-
Functional upregulation of nav1.8 sodium channels on the membrane of dorsal root Ganglia neurons contributes to the development of cancer-induced bone pain.PLoS One. 2014 Dec 11;9(12):e114623. doi: 10.1371/journal.pone.0114623. eCollection 2014. PLoS One. 2014. PMID: 25503076 Free PMC article.
-
TNF-α/STAT3 pathway epigenetically upregulates Nav1.6 expression in DRG and contributes to neuropathic pain induced by L5-VRT.J Neuroinflammation. 2019 Feb 8;16(1):29. doi: 10.1186/s12974-019-1421-8. J Neuroinflammation. 2019. PMID: 30736806 Free PMC article.
-
Modulation of peripheral Na(+) channels and neuronal firing by n-butyl-p-aminobenzoate.Eur J Pharmacol. 2014 Mar 15;727:158-66. doi: 10.1016/j.ejphar.2014.01.036. Epub 2014 Jan 30. Eur J Pharmacol. 2014. PMID: 24486399
-
Regulation/modulation of sensory neuron sodium channels.Handb Exp Pharmacol. 2014;221:111-35. doi: 10.1007/978-3-642-41588-3_6. Handb Exp Pharmacol. 2014. PMID: 24737234 Review.
-
Mini-review - Sodium channels and beyond in peripheral nerve disease: Modulation by cytokines and their effector protein kinases.Neurosci Lett. 2021 Jan 10;741:135446. doi: 10.1016/j.neulet.2020.135446. Epub 2020 Nov 6. Neurosci Lett. 2021. PMID: 33166641 Review.
Cited by
-
Peripheral Mechanism of Cancer-Induced Bone Pain.Neurosci Bull. 2024 Jun;40(6):815-830. doi: 10.1007/s12264-023-01126-6. Epub 2023 Oct 6. Neurosci Bull. 2024. PMID: 37798428 Free PMC article. Review.
-
Molecular targets in bone cancer pain: a systematic review of inflammatory cytokines.J Mol Med (Berl). 2024 Sep;102(9):1063-1088. doi: 10.1007/s00109-024-02464-2. Epub 2024 Jun 28. J Mol Med (Berl). 2024. PMID: 38940936 Free PMC article.
-
Enhanced excitability of cortical neurons in low-divalent solutions is primarily mediated by altered voltage-dependence of voltage-gated sodium channels.Elife. 2021 May 11;10:e67914. doi: 10.7554/eLife.67914. Elife. 2021. PMID: 33973519 Free PMC article.
-
Tetrodotoxin: A New Strategy to Treat Visceral Pain?Toxins (Basel). 2021 Jul 16;13(7):496. doi: 10.3390/toxins13070496. Toxins (Basel). 2021. PMID: 34357968 Free PMC article. Review.
-
Long-lasting analgesia via targeted in situ repression of NaV1.7 in mice.Sci Transl Med. 2021 Mar 10;13(584):eaay9056. doi: 10.1126/scitranslmed.aay9056. Sci Transl Med. 2021. PMID: 33692134 Free PMC article.
References
-
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53:55–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
