Humanising the mouse genome piece by piece
- PMID: 31015419
- PMCID: PMC6478830
- DOI: 10.1038/s41467-019-09716-7
Humanising the mouse genome piece by piece
Abstract
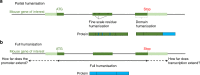
To better understand human health and disease, researchers create a wide variety of mouse models that carry human DNA. With recent advances in genome engineering, the targeted replacement of mouse genomic regions with orthologous human sequences has become increasingly viable, ranging from finely tuned humanisation of individual nucleotides and amino acids to the incorporation of many megabases of human DNA. Here, we examine emerging technologies for targeted genomic humanisation, we review the spectrum of existing genomically humanised mouse models and the insights such models have provided, and consider the lessons learned for designing such models in the future.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

