A suite of phenotypic assays to ensure pipeline diversity when prioritizing drug-like Cryptosporidium growth inhibitors
- PMID: 31015448
- PMCID: PMC6478823
- DOI: 10.1038/s41467-019-09880-w
A suite of phenotypic assays to ensure pipeline diversity when prioritizing drug-like Cryptosporidium growth inhibitors
Abstract
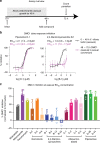
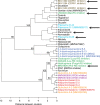
Cryptosporidiosis is a leading cause of life-threatening diarrhea in children, and the only currently approved drug is ineffective in malnourished children and immunocompromised people. Large-scale phenotypic screens are ongoing to identify anticryptosporidial compounds, but optimal approaches to prioritize inhibitors and establish a mechanistically diverse drug development pipeline are unknown. Here, we present a panel of medium-throughput mode of action assays that enable testing of compounds in several stages of the Cryptosporidium life cycle. Phenotypic profiles are given for thirty-nine anticryptosporidials. Using a clustering algorithm, the compounds sort by phenotypic profile into distinct groups of inhibitors that are either chemical analogs (i.e. same molecular mechanism of action (MMOA)) or known to have similar MMOA. Furthermore, compounds belonging to multiple phenotypic clusters are efficacious in a chronic mouse model of cryptosporidiosis. This suite of phenotypic assays should ensure a drug development pipeline with diverse MMOA without the need to identify underlying mechanisms.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Phenotypic screening techniques for Cryptosporidium drug discovery.Expert Opin Drug Discov. 2021 Jan;16(1):59-74. doi: 10.1080/17460441.2020.1812577. Epub 2020 Sep 7. Expert Opin Drug Discov. 2021. PMID: 32892652 Review.
-
A high-throughput phenotypic screen identifies clofazimine as a potential treatment for cryptosporidiosis.PLoS Negl Trop Dis. 2017 Feb 3;11(2):e0005373. doi: 10.1371/journal.pntd.0005373. eCollection 2017 Feb. PLoS Negl Trop Dis. 2017. PMID: 28158186 Free PMC article.
-
Cryptosporidiosis Drug Discovery: Opportunities and Challenges.ACS Infect Dis. 2016 Aug 12;2(8):530-7. doi: 10.1021/acsinfecdis.6b00094. Epub 2016 Jul 22. ACS Infect Dis. 2016. PMID: 27626293 Review.
-
Identification of a potent benzoxaborole drug candidate for treating cryptosporidiosis.Nat Commun. 2019 Jun 27;10(1):2816. doi: 10.1038/s41467-019-10687-y. Nat Commun. 2019. PMID: 31249291 Free PMC article.
-
A Novel Piperazine-Based Drug Lead for Cryptosporidiosis from the Medicines for Malaria Venture Open-Access Malaria Box.Antimicrob Agents Chemother. 2018 Mar 27;62(4):e01505-17. doi: 10.1128/AAC.01505-17. Print 2018 Apr. Antimicrob Agents Chemother. 2018. PMID: 29339392 Free PMC article.
Cited by
-
Activity of (1-benzyl-4-triazolyl)-indole-2-carboxamides against Toxoplasma gondii and Cryptosporidium parvum.Int J Parasitol Drugs Drug Resist. 2022 Aug;19:6-20. doi: 10.1016/j.ijpddr.2022.04.001. Epub 2022 Apr 16. Int J Parasitol Drugs Drug Resist. 2022. PMID: 35462232 Free PMC article.
-
Tyrosine Kinase Inhibitors Display Potent Activity against Cryptosporidium parvum.Microbiol Spectr. 2023 Feb 14;11(1):e0387422. doi: 10.1128/spectrum.03874-22. Epub 2022 Dec 19. Microbiol Spectr. 2023. PMID: 36533912 Free PMC article.
-
Cryptosporidium PI(4)K inhibitor EDI048 is a gut-restricted parasiticidal agent to treat paediatric enteric cryptosporidiosis.Nat Microbiol. 2024 Nov;9(11):2817-2835. doi: 10.1038/s41564-024-01810-x. Epub 2024 Oct 8. Nat Microbiol. 2024. PMID: 39379634 Free PMC article.
-
Cryptosporidium: Host-Parasite Interactions and Pathogenesis.Curr Clin Microbiol Rep. 2021;8(2):62-67. doi: 10.1007/s40588-021-00159-7. Epub 2021 Feb 8. Curr Clin Microbiol Rep. 2021. PMID: 33585166 Free PMC article. Review.
-
Safe and effective treatments are needed for cryptosporidiosis, a truly neglected tropical disease.BMJ Glob Health. 2023 Aug;8(8):e012540. doi: 10.1136/bmjgh-2023-012540. BMJ Glob Health. 2023. PMID: 37541693 Free PMC article. No abstract available.
References
-
- Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545–1602. doi: 10.1016/S0140-6736(16)31678-6. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

