Meta-analysis of epigenome-wide association studies in neonates reveals widespread differential DNA methylation associated with birthweight
- PMID: 31015461
- PMCID: PMC6478731
- DOI: 10.1038/s41467-019-09671-3
Meta-analysis of epigenome-wide association studies in neonates reveals widespread differential DNA methylation associated with birthweight
Abstract
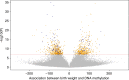
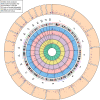
Birthweight is associated with health outcomes across the life course, DNA methylation may be an underlying mechanism. In this meta-analysis of epigenome-wide association studies of 8,825 neonates from 24 birth cohorts in the Pregnancy And Childhood Epigenetics Consortium, we find that DNA methylation in neonatal blood is associated with birthweight at 914 sites, with a difference in birthweight ranging from -183 to 178 grams per 10% increase in methylation (PBonferroni < 1.06 x 10-7). In additional analyses in 7,278 participants, <1.3% of birthweight-associated differential methylation is also observed in childhood and adolescence, but not adulthood. Birthweight-related CpGs overlap with some Bonferroni-significant CpGs that were previously reported to be related to maternal smoking (55/914, p = 6.12 x 10-74) and BMI in pregnancy (3/914, p = 1.13x10-3), but not with those related to folate levels in pregnancy. Whether the associations that we observe are causal or explained by confounding or fetal growth influencing DNA methylation (i.e. reverse causality) requires further research.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Lawlor DA, et al. Association of existing diabetes, gestational diabetes and glycosuria in pregnancy with macrosomia and offspring body mass index, waist and fat mass in later childhood: Findings from a prospective pregnancy cohort. Diabetologia. 2010;53:89–97. doi: 10.1007/s00125-009-1560-z. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MC_U123292700/MRC_/Medical Research Council/United Kingdom
- R24 ES028507/ES/NIEHS NIH HHS/United States
- MC_UU_00011/1/MRC_/Medical Research Council/United Kingdom
- MC_EX_MR/M01424X/1/MRC_/Medical Research Council/United Kingdom
- R01 ES021369/ES/NIEHS NIH HHS/United States
- R01 ES013744/ES/NIEHS NIH HHS/United States
- R01 MD013299/MD/NIMHD NIH HHS/United States
- R24 ES028522/ES/NIEHS NIH HHS/United States
- R01 DK085173/DK/NIDDK NIH HHS/United States
- MR/S003886/1/MRC_/Medical Research Council/United Kingdom
- MR/P012019/1/MRC_/Medical Research Council/United Kingdom
- MC_UU_00026/3/MRC_/Medical Research Council/United Kingdom
- R00 ES023450/ES/NIEHS NIH HHS/United States
- R24 ES028529/ES/NIEHS NIH HHS/United States
- UH3 OD023275/OD/NIH HHS/United States
- UG3 OD023356/OD/NIH HHS/United States
- R21 ES028226/ES/NIEHS NIH HHS/United States
- P30 ES023515/ES/NIEHS NIH HHS/United States
- R01 ES025574/ES/NIEHS NIH HHS/United States
- R01 ES022223/ES/NIEHS NIH HHS/United States
- MC_UU_00011/6/MRC_/Medical Research Council/United Kingdom
- R24 ES028531/ES/NIEHS NIH HHS/United States
- R01 DK103246/DK/NIDDK NIH HHS/United States
- P30 ES019776/ES/NIEHS NIH HHS/United States
- MC_UU_00011/5/MRC_/Medical Research Council/United Kingdom
- MC_U123292701/MRC_/Medical Research Council/United Kingdom
- R01 ES025531/ES/NIEHS NIH HHS/United States
- P01 HL132825/HL/NHLBI NIH HHS/United States
- 001/WHO_/World Health Organization/International
- R01 HD034568/HD/NICHD NIH HHS/United States
- MR/S019669/1/MRC_/Medical Research Council/United Kingdom
- R01 ES022934/ES/NIEHS NIH HHS/United States
- R01 AI091905/AI/NIAID NIH HHS/United States
- MC_UU_12013/2/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Medical

