Risks of budesonide/formoterol for the treatment of stable COPD: a meta-analysis
- PMID: 31015757
- PMCID: PMC6448539
- DOI: 10.2147/COPD.S192166
Risks of budesonide/formoterol for the treatment of stable COPD: a meta-analysis
Abstract
Purpose: The aim of this study was to investigate the comparative risks of budesonide/formoterol, versus placebo or monotherapies, for the treatment of patients with stable COPD.
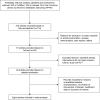
Materials and methods: We undertook a systematic search of the literature in PubMed, Embase, and the Cochrane Central Register of Controlled Trials, for randomized controlled trials (RCTs) comparing budesonide/formoterol with control regimens for the treatment of patients with stable COPD and at least 12 weeks of follow-up, meeting the inclusion criteria. Studies were reviewed, and OR with corresponding 95% CI was used to pool the results.
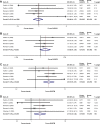
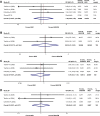
Results: A total of eight studies involving 9,254 patients met the inclusion criteria of this meta-analysis. Compared with placebo, combination therapy with budesonide/formoterol was associated with a significantly higher risk of adverse effects including oral candidiasis (OR: 3.09, 95% CI: 1.95-4.91) and dysphonia (OR: 2.76, 95% CI: 1.40-5.44), but not pneumonia (OR: 0.94, 95% CI: 0.64-1.37) or bronchitis (OR: 1.36, 95% CI: 0.95-1.95). A similar pattern was also evident for the comparison of formoterol with budesonide/formoterol, with increased occurrence of oral candidiasis (OR: 2.72, 95% CI: 1.33-5.58) and dysphonia (OR: 4.13, 95% CI: 1.95-8.76); however, there were no significant differences in pneumonia (OR: 1.31, 95% CI: 0.98-1.74) or bronchitis (OR: 1.05, 95% CI: 0.83-1.31). In contrast, compared with budesonide, combined budesonide/formoterol was associated with similar risks of adverse effects, including pneumonia (OR: 1.20, 95% CI: 0.60-2.39), bronchitis (OR: 0.95, 95% CI: 0.41-2.20), oral candidiasis (OR: 0.79, 95% CI: 0.41-1.53), and dysphonia (OR: 1.00, 95% CI: 0.40-2.47).
Conclusion: Combination therapy does not cause more adverse events, including pneumonia and bronchitis, than control (placebo, formoterol, or budesonide) treatment in patients with stable COPD, while there were higher risks of oral candidiasis and dysphonia compared with the non-inhaled corticosteroid group (placebo, formoterol).
Keywords: COPD; budesonide/formoterol; meta-analysis; randomized controlled trial; risk.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

