Biliary tract cancers: current knowledge, clinical candidates and future challenges
- PMID: 31015767
- PMCID: PMC6446989
- DOI: 10.2147/CMAR.S157092
Biliary tract cancers: current knowledge, clinical candidates and future challenges
Abstract
Biliary tract cancers (BTCs) are rare with poor prognosis. Due to the advent of genomic sequencing, new data have emerged regarding the molecular makeup of this disease. To add to the complexity, various subtypes also harbor a varied genetic composition. The commonly mutated genes associated with this cancer are KRAS, EGFR, IDH, FGFR and BAP1. Various clinical studies are looking at targeting these genetic mutations. Another therapeutic area of note is the potential for the use of immunotherapy in patients with BTC. Although BTC may be a result of chronic inflammation, this does not necessarily translate into increased immunogenicity. This literature review discusses the diverse molecular and immune-related pathways in patients with BTC and their potential therapeutic implications.
Keywords: biliary tract cancer; extrahepatic cholangiocarci-noma; gallbladder cancer; genome sequencing; immunotherapy; intrahepatic cholangiocarcinoma; molecular targets.
Conflict of interest statement
Disclosure Noor-ul-Ain Tariq received honoraria for lecturers, participation in writing guidelines and travel reimbursements in 2014 from Boehringer Ingelheim and received funding from the Timpson fellowship. Boehringer Ingelheim or Timpson have no influence over the contents of this review. Mairéad G McNamara was advisory board member of Ipsen, SHIRE, Celgene and Sirtex, received research support from NuCana BioMed Ltd. and SHIRE, received honoraria for participation in Speaker’s Bureau from Pfizer and Ipsen and received travel expenses from Bayer. Juan W Valle received travel grants from Celgene, Ipsen, Novartis, NuCana for more than 5 years, received honoraria for participation in Speakers’ Bureau for Abbott, Celgene, Ipsen, Novartis, Pfizer and Sir-tex and provided consulting or advisory role for for Abbott, Agios, AstraZeneca, Baxalta, Bioven, Celgene, Delcath, Genoscience Pharma, Incyte, Ipsen, Keocyt, Lilly, Merck, MidaTech, Mundipharma, Novartis, NuCana, PCI Biotech, Pfizer, Pieris Pharmaceuticals and QED Pharmaceuticals. The authors report no other conflicts of interest in this work.
Figures
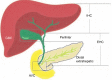

References
-
- de Groen PC, Gores GJ, Larusso NF, Gunderson LL, Nagorney DM. Biliary tract cancers. N Engl J Med Overseas Ed. 1999;341(18):1368–1378. - PubMed
-
- Charbel H, Al-Kawas FH. Cholangiocarcinoma: epidemiology, risk factors, pathogenesis, and diagnosis. Curr Gastroenterol Rep. 2011;13(2):182–187. - PubMed
-
- England Public Health . National Cancer Intelligence Network: Rare and Less Common Cancers, Incidence and Mortality in England. London: 2015.
-
- Cancer.gov Surveillance epidemiology and end results program. 2015 Seer Data. [Accessed February 20, 2019]. Available from: https://seer.cancer.gov/statfacts/html/livibd.html.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

