Diversity of PEGylation methods of liposomes and their influence on RNA delivery
- PMID: 31015904
- PMCID: PMC6457174
- DOI: 10.1039/c8md00515j
Diversity of PEGylation methods of liposomes and their influence on RNA delivery
Abstract
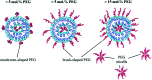
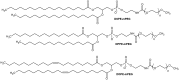
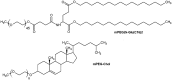
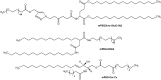
Gene therapy is a promising approach for personalized medicine, but its application in humans requires development of efficient and safe vehicles. PEGylated liposomes are some of the most suitable delivery systems for nucleic acids because of their stability under physiological conditions and prolonged circulation time, compared to conventional and other types of "stealth" liposomes. In vitro/in vivo activity of PEGylated liposomes is highly dependent on PEG motif abundance. The process of "stealth" coverage formation is a very important parameter for efficient transfection assays and further fate determination of the PEG layer after tissue penetration. In this review, we discuss the latest methods of PEGylated liposome preparation.
Figures



Similar articles
-
Post-pegylated lipoplexes are promising vehicles for gene delivery in RPE cells.J Control Release. 2007 Aug 28;121(3):208-17. doi: 10.1016/j.jconrel.2007.05.033. Epub 2007 Jun 5. J Control Release. 2007. PMID: 17630013
-
Anti-PEG IgM Production via a PEGylated Nanocarrier System for Nucleic Acid Delivery.Methods Mol Biol. 2019;1943:333-346. doi: 10.1007/978-1-4939-9092-4_22. Methods Mol Biol. 2019. PMID: 30838627
-
PEGylated galactosylated cationic liposomes for hepatocytic gene delivery.Colloids Surf B Biointerfaces. 2014 Oct 1;122:482-490. doi: 10.1016/j.colsurfb.2014.07.010. Epub 2014 Jul 15. Colloids Surf B Biointerfaces. 2014. PMID: 25096720
-
From conventional to stealth liposomes: a new frontier in cancer chemotherapy.Tumori. 2003 May-Jun;89(3):237-49. doi: 10.1177/030089160308900302. Tumori. 2003. PMID: 12908776 Review.
-
PEGylation of proteins and liposomes: a powerful and flexible strategy to improve the drug delivery.Curr Drug Metab. 2012 Jan;13(1):105-19. doi: 10.2174/138920012798356934. Curr Drug Metab. 2012. PMID: 21892917 Review.
Cited by
-
Approaches to Address PK-PD Challenges of Conventional Liposome Formulation with Special Reference to Cancer, Alzheimer's, Diabetes, and Glaucoma: An Update on Modified Liposomal Drug Delivery System.Curr Drug Metab. 2022;23(9):678-692. doi: 10.2174/1389200223666220609141459. Curr Drug Metab. 2022. PMID: 35692131 Review.
-
The Art of PEGylation: From Simple Polymer to Sophisticated Drug Delivery System.Int J Mol Sci. 2025 Mar 27;26(7):3102. doi: 10.3390/ijms26073102. Int J Mol Sci. 2025. PMID: 40243857 Free PMC article. Review.
-
Targeted Liposomal Drug Delivery: Overview of the Current Applications and Challenges.Life (Basel). 2024 May 24;14(6):672. doi: 10.3390/life14060672. Life (Basel). 2024. PMID: 38929656 Free PMC article. Review.
-
Nanoliposomes as nonviral vectors in cancer gene therapy.MedComm (2020). 2024 Jun 25;5(7):e583. doi: 10.1002/mco2.583. eCollection 2024 Jul. MedComm (2020). 2024. PMID: 38919334 Free PMC article. Review.
-
Lipid-Coated Polymeric Nanoparticles for the Photodynamic Therapy of Head and Neck Squamous Cell Carcinomas.Pharmaceutics. 2023 Oct 2;15(10):2412. doi: 10.3390/pharmaceutics15102412. Pharmaceutics. 2023. PMID: 37896172 Free PMC article.
References
-
- Fire A., Xu S., Montgomery M. K., Kostas S. A., Driver S. E., Mello C. C. Nature. 1998;391:806–811. - PubMed
-
- Khaitov M. R., Shilovskiy I. P., Nikonova A. A., Shershakova N. N., Kamyshnikov O. Y., Babakhin A. A., Hum. Gene Ther., 2014, 257 , 642 –650 , , Available from: http://www.ncbi.nlm.nih.gov/pubmed/24655063 . - PubMed
-
- Ashfaq U. A., Yousaf M. Z., Aslam M., Ejaz R., Jahan S., Ullah O., Virol J., 2011, 8 , 276 –282 , , Available from: http://virologyj.biomedcentral.com/articles/10.1186/1743-422X-8-276 . - PMC - PubMed
-
- Hoerter J. A. H., Walter N. G., RNA, 2007, 13 , 1887 –1893 , , Available from: http://www.rnajournal.org/cgi/doi/10.1261/rna.602307 . - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

