Rhodium(iii)-catalyzed diverse [4 + 1] annulation of arenes with 1,3-enynes via sp3/sp2 C-H activation and 1,4-rhodium migration
- PMID: 31015939
- PMCID: PMC6457175
- DOI: 10.1039/c9sc00545e
Rhodium(iii)-catalyzed diverse [4 + 1] annulation of arenes with 1,3-enynes via sp3/sp2 C-H activation and 1,4-rhodium migration
Abstract
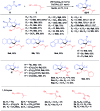
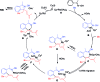
Nitrogen-rich heterocyclic compounds have a profound impact on human health. Despite the numerous synthetic methods, diversified, step-economic, and general synthesis of heterocycles remains limited. C-H bond functionalization catalyzed by rhodium(iii) cyclopentadienyls has proven to be a powerful strategy in the synthesis of diversified heterocycles. Herein we describe rhodium(iii)-catalyzed sp2 and sp3 C-H activation-oxidative annulations between aromatic substrates and 1,3-enynes, where alkenyl-to-allyl 1,4-rhodium(iii) migration enabled the generation of electrophilic rhodium(iii) π-allyls via remote C-H functionalization. Subsequent nucleophilic trapping of these species by various sp2-hybridized N-nucleophiles delivered three classes (external salts, inner salts, and neutral azacycles) of five-membered azacycles bearing a tetrasubstituted saturated carbon center, as a result of [4 + 1] annulation with the alkyne being a one-carbon synthon. All the reactions proceeded under relatively mild conditions with broad substrate scope, high efficiency, and excellent regioselectivity. The synthetic applications of this protocol have also been demonstrated, and experimental studies have been performed to support the proposed mechanism.
Figures






Similar articles
-
One-Carbon Oxidative Annulations of 1,3-Enynes by Catalytic C-H Functionalization and 1,4-Rhodium(III) Migration.Chemistry. 2018 Mar 15;24(16):4050-4054. doi: 10.1002/chem.201706043. Epub 2018 Feb 21. Chemistry. 2018. PMID: 29356188
-
Rhodium(III)-Catalyzed Asymmetric [4+1] and [5+1] Annulation of Arenes and 1,3-Enynes: A Distinct Mechanism of Allyl Formation and Allyl Functionalization.Angew Chem Int Ed Engl. 2020 Dec 7;59(50):22706-22713. doi: 10.1002/anie.202010832. Epub 2020 Oct 7. Angew Chem Int Ed Engl. 2020. PMID: 32886841
-
All-Carbon [3+3] Oxidative Annulations of 1,3-Enynes by Rhodium(III)-Catalyzed C-H Functionalization and 1,4-Migration.Angew Chem Int Ed Engl. 2015 Aug 17;54(34):9958-62. doi: 10.1002/anie.201503978. Epub 2015 Jul 15. Angew Chem Int Ed Engl. 2015. PMID: 26224377 Free PMC article.
-
Rhodium-Catalyzed C(sp2 )- or C(sp3 )-H Bond Functionalization Assisted by Removable Directing Groups.Angew Chem Int Ed Engl. 2019 Jun 17;58(25):8304-8329. doi: 10.1002/anie.201808159. Epub 2019 Apr 1. Angew Chem Int Ed Engl. 2019. PMID: 30311719 Review.
-
Imidates: an emerging synthon for N-heterocycles.Org Biomol Chem. 2019 Nov 27;17(46):9829-9843. doi: 10.1039/c9ob01899a. Org Biomol Chem. 2019. PMID: 31720673 Review.
Cited by
-
Recent Advancements in the Cyclization Strategies of 1,3-Enynes Towards the Synthesis of Heterocyclic/Carbocyclic Frameworks.Chem Asian J. 2025 Apr 17;20(8):e202401657. doi: 10.1002/asia.202401657. Epub 2025 Mar 4. Chem Asian J. 2025. PMID: 39976556 Free PMC article. Review.
-
Chemical insights into the synthetic chemistry of five-membered saturated heterocycles-a transition metal-catalyzed approach.Front Chem. 2023 Jul 26;11:1185669. doi: 10.3389/fchem.2023.1185669. eCollection 2023. Front Chem. 2023. PMID: 37564110 Free PMC article. Review.
-
Divergent Pd-catalyzed cross-coupling of allenyloxazolidinones to give chiral 1,3-dienes and vinyloxazolidinones.Chem Sci. 2019 Aug 9;10(39):9051-9056. doi: 10.1039/c9sc03215k. eCollection 2019 Oct 21. Chem Sci. 2019. PMID: 31827747 Free PMC article.
-
Rh(iii)-catalyzed sp3/sp2-C-H heteroarylations via cascade C-H activation and cyclization.Chem Sci. 2024 Mar 27;15(17):6544-6551. doi: 10.1039/d3sc06955a. eCollection 2024 May 1. Chem Sci. 2024. PMID: 38699273 Free PMC article.
-
Chemodivergent assembly of ortho-functionalized phenols with tunable selectivity via rhodium(III)-catalyzed and solvent-controlled C-H activation.Commun Chem. 2021 Jun 3;4(1):81. doi: 10.1038/s42004-021-00518-x. Commun Chem. 2021. PMID: 36697536 Free PMC article.
References
-
- Ritleng V., Sirlin C., Pfeffer M. Chem. Rev. 2002;102:1731–1770. - PubMed
- Daugulis O., Do H.-Q., Shabashov D. Acc. Chem. Res. 2009;42:1074–1086. - PMC - PubMed
- Chen X., Engle K. M., Wang D. H., Yu J. Q. Angew. Chem. 2009;48:5094–5115. - PMC - PubMed
- Colby D. A., Bergman R. G., Ellman J. A. Chem. Rev. 2010;110:624–655. - PMC - PubMed
- Lyons T. W., Sanford M. S. Chem. Rev. 2011;110:1147–1169. - PMC - PubMed
- Yeung C. S., Dong V. M. Chem. Rev. 2011;111:1215–1292. - PubMed
- Cho S. H., Kim J. Y., Kwak J., Chang S. Chem. Soc. Rev. 2011;40:5068–5083. - PubMed
- Ackermann L. Chem. Rev. 2011;111:1315–1345. - PubMed
- Wencel-Delord J., Droge T., Liu F., Glorius F. Chem. Soc. Rev. 2011;40:4740–4761. - PubMed
- Gutekunst W. R., Baran P. S. Chem. Soc. Rev. 2011;40:1976–1991. - PubMed
- McMurray L., O'Hara F., Gaunt M. J. Chem. Soc. Rev. 2011;40:1885–1898. - PubMed
- Yamaguchi J., Yamaguchi A. D., Itami K. Angew. Chem., Int. Ed. 2012;51:8960–9009. - PubMed
- Li B.-J., Shi Z.-J. Chem. Soc. Rev. 2014;41:5588–5598. - PubMed
- Zheng C., You S.-L. RSC Adv. 2014;4:6173–6214.
- Guo X.-X., Gu D.-W., Wu Z.-X., Zhang W.-B. Chem. Rev. 2015;115:1622–1651. - PubMed
- Yang L., Huang H. M. Chem. Rev. 2015;115:3468–3517. - PubMed
- Gensch T., Hopkinson M. N., Glorius F., Wencel-Delord J. Chem. Soc. Rev. 2016;45:2900–2936. - PubMed
-
- Yang Y., Li K., Cheng Y., Wan D., Li M., You J. Chem. Commun. 2016;52:2872–2884. - PubMed
- Piou T., Rovis T. Acc. Chem. Res. 2018;51:170–180. - PMC - PubMed
- Guimond N., Gouliaras C., Fagnou K. J. Am. Chem. Soc. 2010;132:6908–6909. - PubMed
- Patureau F. W., Besset T., Kuhl N., Glorius F. J. Am. Chem. Soc. 2011;133:2154–2156. - PubMed
- Li B. J., Wang H. Y., Zhu Q. L., Shi Z. J. Angew. Chem., Int. Ed. 2012;51:3948–3952. - PubMed
- Zhou M.-B., Pi R., Hu M., Yang Y., Song R. J., Xia Y. Z., Li J. H. Angew. Chem., Int. Ed. 2014;53:11338–11341. - PubMed
- Zhou T., Wang Y. W., Li B., Wang B. Q. Org. Lett. 2016;18:5066–5069. - PubMed
- Stemmler R. T., Bolm C. Adv. Synth. Catal. 2007;349:1185–1198.
-
- Satoh T., Miura M. Chem.–Eur. J. 2010;16:11212–11222. - PubMed
- Satoh T., Miura M. Org. Lett. 2007;9:1407–1409. - PubMed
- Colby D. A., Bergman R. G., Ellman J. A. J. Am. Chem. Soc. 2008;130:3645–3651. - PMC - PubMed
- Li L., Brennessel W. W., Jones W. D. J. Am. Chem. Soc. 2008;130:12414–12419. - PubMed
- Stuart D. R., Bertrand-Laperle M., Burgess K. M. N., Fagnou K. J. Am. Chem. Soc. 2008;130:16474–16475. - PubMed
- Umeda N., Tsurugi H., Satoh T., Miura M. Angew. Chem., Int. Ed. 2008;47:4019–4022. - PubMed
- Guimond N., Fagnou K. J. Am. Chem. Soc. 2009;131:12050–12051. - PubMed
- Too P. C., Wang Y.-F., Chiba S. Org. Lett. 2010;12:5688–5691. - PubMed
- Stuart D. R., Alsabeh P., Kuhn M., Fagnou K. J. Am. Chem. Soc. 2010;132:18326–18339. - PubMed
- Muralirajan K., Parthasarathy K., Cheng C.-H. Angew. Chem., Int. Ed. 2011;50:4169–4172. - PubMed
- Umeda N., Hirano K., Satoh T., Shibata N., Sato H., Miura M. J. Org. Chem. 2011;76:13–24. - PubMed
- Yan H., Wang H.-L., Li X.-C., Xin X.-Y., Wang C.-X., Wan B.-S. Angew. Chem., Int. Ed. 2015;54:10613–10617. - PubMed
- Huestis M. P., Chan L., Stuart D. R., Fagnou K. Angew. Chem., Int. Ed. 2011;50:1338–1341. - PubMed
- Ghoraia D., Choudhury J. Chem. Commun. 2014;50:15159–15162. - PubMed
- Ghorai D., Choudhury J. ACS Catal. 2015;5:2692–2696.
- Davies D. L., Ellul C. E., Macgregor S. A., McMullin C. L., Singh K. J. Am. Chem. Soc. 2015;137:9659–9669. - PubMed
- Gandeepan P., Cheng C. -H. Chem.–Asian J. 2016;11:448–546. - PubMed
- Mascareñas J. L. Angew. Chem., Int. Ed. 2016;55:11000–11019. - PubMed
- Wang C.-Q., Ye L., Feng C., Loh T.-P. J. Am. Chem. Soc. 2017;139:1762–1765. - PubMed
-
- Burns D. J., Lam H. W. Angew. Chem., Int. Ed. 2014;53:9931–9935. - PMC - PubMed
- Burns D. J., Best D., Wieczysty M. D., Lam H. W. Angew. Chem., Int. Ed. 2015;54:9958–9962. - PMC - PubMed
- Dooley J. D., Lam H. W. Chem.–Eur. J. 2018;24:4050–4054. - PubMed
- Korkis S. E., Burns D. J., Lam H. W. J. Am. Chem. Soc. 2016;138:12252–12257. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

