Monodisperse polysarcosine-based highly-loaded antibody-drug conjugates
- PMID: 31015945
- PMCID: PMC6457330
- DOI: 10.1039/c9sc00285e
Monodisperse polysarcosine-based highly-loaded antibody-drug conjugates
Abstract
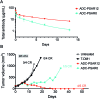
Antibody-drug conjugates (ADCs) convey highly potent anticancer drugs to antigen-expressing tumor cells, thereby sparing healthy tissues throughout the body. Pharmacokinetics and tolerability of ADCs are predominantly influenced by the drug-antibody ratio (DAR) of the conjugates, which is to-date limited to a value of 3-4 drugs per antibody in ADCs under clinical investigations. Here, we report the synthesis of monodisperse (i.e. discrete) polysarcosine compounds and their use as a hydrophobicity masking entity for the construction of highly-loaded homogeneous β-glucuronidase-responsive antibody-drug conjugates (ADCs). The highly hydrophilic drug-linker platform described herein improves drug-loading, physicochemical properties, pharmacokinetics and in vivo antitumor efficacy of the resulting conjugates.
Figures




References
-
- Beck A., Goetsch L., Dumontet C., Corvaïa N. Nat. Rev. Drug Discovery. 2017;16:315–337. - PubMed
-
- Innovations for Next-Generation Antibody-Drug Conjugates, ed. M. Damelin, Humana Press, 2018th edn, 2018.
-
- Wade H., ADC Beacon [database], https://beacon-intelligence.com, 2018.
-
- Chari R. V. J., Miller M. L., Widdison W. C. Angew. Chem., Int. Ed. 2014;53:3796–3827. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

