Proteomic analysis of human mesenchymal stromal cell secretomes: a systematic comparison of the angiogenic potential
- PMID: 31016031
- PMCID: PMC6467904
- DOI: 10.1038/s41536-019-0070-y
Proteomic analysis of human mesenchymal stromal cell secretomes: a systematic comparison of the angiogenic potential
Abstract
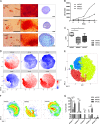
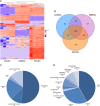
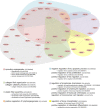
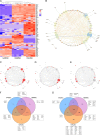
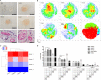
Human mesenchymal stromal cell (hMSC) secretomes have shown to influence the microenvironment upon injury, promoting cytoprotection, angiogenesis, and tissue repair. The angiogenic potential is of particular interest for the treatment of ischemic diseases. Interestingly, hMSC secretomes isolated from different tissue sources have shown dissimilarities with respect to their angiogenic profile. This study compares angiogenesis of hMSC secretomes from adipose tissue (hADSCs), bone marrow (hBMSCs), and umbilical cord Wharton's jelly (hWJSCs). hMSC secretomes were obtained under xenofree conditions and analyzed by liquid chromatography tandem mass spectrometry (LC/MS-MS). Biological processes related to angiogenesis were found to be enriched in the proteomic profile of hMSC secretomes. hWJSC secretomes revealed a more complete angiogenic network with higher concentrations of angiogenesis related proteins, followed by hBMSC secretomes. hADSC secretomes lacked central angiogenic proteins and expressed most detected proteins to a significantly lower level. In vivo all secretomes induced vascularization of subcutaneously implanted Matrigel plugs in mice. Differences in secretome composition were functionally analyzed with monocyte and endothelial cell (EC) in vitro co-culture experiments using vi-SNE based multidimensional flow cytometry data analysis. Functional responses between hBMSC and hWJSC secretomes were comparable, with significantly higher migration of CD14++ CD16- monocytes and enhanced macrophage differentiation compared with hADSC secretomes. Both secretomes also induced a more profound pro-angiogenic phenotype of ECs. These results suggest hWJSCs secretome as the most potent hMSC source for inflammation-mediated angiogenesis induction, while the potency of hADSC secretomes was lowest. This systematic analysis may have implication on the selection of hMSCs for future clinical studies.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
LinkOut - more resources
Full Text Sources
Medical
Research Materials

