Chronic NO Restriction in Hypertensive Rats Increases Abdominal but Not Thoracic Aortic Intrinsic Stiffness via an Augmentation in Profibrotic Materials
- PMID: 31016040
- PMCID: PMC6444237
- DOI: 10.1155/2019/8070198
Chronic NO Restriction in Hypertensive Rats Increases Abdominal but Not Thoracic Aortic Intrinsic Stiffness via an Augmentation in Profibrotic Materials
Abstract
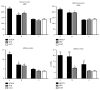
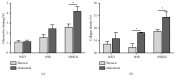
The spontaneously hypertensive rat model with reduced NO synthesis (SHRLN) shares features with aging and hypertension in humans, among other a severe aortic stiffening. The present in vivo study aimed to compare thoracic (TA) and abdominal (AA) aortic stiffness in the SHRLN (treated 5 weeks with L-NAME), SHR, and normotensive Wistar Kyoto (WKY). Dynamic properties of TA and AA were measured in the same rats, using echotracking recording of aortic diameter coupled with blood pressure (BP). Measurements were performed first at operating BP and then after BP reduction in hypertensive rats, thus in isobaric conditions. Histological staining and immunohistochemistry were used for structural analysis at both sites. At operating pressure, BP and pulse pressure (PP) were higher in SHRLN compared with SHR. Stiffness index was also increased and distensibility decreased in both TA and AA in SHRLN. At WKY-matched blood pressure, isobaric AA parameters remained specifically altered in SHRLN, whereas TA recovered to values identical to WKYs. Collagen, fibronectin, α5-selectin, and FAK were increased in SHRLN compared with SHR or WKY. Nevertheless, only the strong accumulations of fibronectin and collagen at the AA site in SHRLN were associated with intrinsic stiffening. In conclusion, we confirm that NO restriction associated with hypertension induces a severe pathological phenotype and shows that L-NAME induced stiffening is more pronounced in AA than in TA as a result of greater fibrosis.
Figures


References
LinkOut - more resources
Full Text Sources
Miscellaneous

