Inhibition of CaMKIIα Activity Enhances Antitumor Effect of Fullerene C60 Nanocrystals by Suppression of Autophagic Degradation
- PMID: 31016106
- PMCID: PMC6468974
- DOI: 10.1002/advs.201801233
Inhibition of CaMKIIα Activity Enhances Antitumor Effect of Fullerene C60 Nanocrystals by Suppression of Autophagic Degradation
Abstract
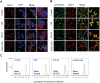
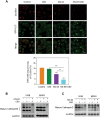
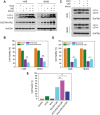
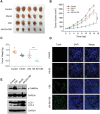
Fullerene C60 nanocrystals (nano-C60) possess various attractive bioactivities, including autophagy induction and calcium/calmodulin-dependent protein kinase IIα (CaMKIIα) activation. CaMKIIα is a multifunctional protein kinase involved in many cellular processes including tumor progression; however, the biological effects of CaMKIIα activity modulated by nano-C60 in tumors have not been reported, and the relationship between CaMKIIα activity and autophagic degradation remains unclear. Herein, nano-C60 is demonstrated to elicit reactive oxygen species (ROS)-dependent cytotoxicity and persistent activation of CaMKIIα in osteosarcoma (OS) cells. CaMKIIα activation, in turn, produces a protective effect against cytotoxicity from nano-C60 itself. Inhibition of CaMKIIα activity by either the chemical inhibitor KN-93 or CaMKIIα knockdown dramatically promotes the anti-OS effect of nano-C60. Moreover, inhibition of CaMKIIα activity causes lysosomal alkalinization and enlargement, and impairs the degradation function of lysosomes, leading to autophagosome accumulation. Importantly, excessive autophagosome accumulation and autophagic degradation blocking are shown to play an important role in KN-93-enhanced-OS cell death. The synergistic anti-OS efficacy of KN-93 and nano-C60 is further revealed in an OS-xenografted murine model. The results demonstrate that CaMKIIα inhibition, along with the suppression of autophagic degradation, presents a promising strategy for improving the antitumor efficacy of nano-C60.
Keywords: autophagic degradation; autophagy; calcium/calmodulin‐dependent protein kinase IIα; fullerene C60 nanocrystals; osteosarcoma therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Zhao R., Han X., Li Y., Wang H., Ji T., Zhao Y., Nie G., ACS Nano 2017, 11, 8103. - PubMed
-
- Tong S., Quinto C. A., Zhang L., Mohindra P., Bao G., ACS Nano 2017, 11, 6808. - PubMed
-
- Riedinger A., Avellini T., Curcio A., Asti M., Xie Y., Tu R., Marras S., Lorenzoni A., Rubagotti S., Iori M., Capponi P. C., Versari A., Manna L., Seregni E., Pellegrino T., J. Am. Chem. Soc. 2015, 137, 15145. - PubMed
LinkOut - more resources
Full Text Sources
