Adverse Effect Profile and Effectiveness of Sodium Glucose Co-transporter 2 Inhibitors (SGLT2i) - A Prospective Real-world Setting Study
- PMID: 31016153
- PMCID: PMC6446693
- DOI: 10.4103/ijem.IJEM_566_18
Adverse Effect Profile and Effectiveness of Sodium Glucose Co-transporter 2 Inhibitors (SGLT2i) - A Prospective Real-world Setting Study
Abstract
Background: Clinical trials have shown promising results in terms of glycemic control and weight reduction with the use of sodium glucose co-transporter 2 inhibitors (SGLT2i) in type 2 diabetes mellitus (T2DM). However, real-world evidence from standard clinical practice especially from Asia is still limited. The aim of this study was to evaluate the safety and effectiveness of SGLT2i in patients with T2DM in real-world setting.
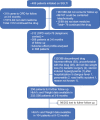
Methods: This was a prospective observational longitudinal study involving consecutive patients with T2DM, initiated on SGLT2i from 1 April 2015 to 31 March 2016. The adverse effects and metabolic parameters were evaluated at 3 monthly intervals up to 1 year.
Results: Total 486 patients were initiated on SGLT2i. At baseline, mean age, glycosylated haemoglobin (HbA1c), and weight was 51.03 ± 9.82 years, 8.76 ± 1.59%, and 89.32 ± 16.04 kg, respectively. Data of 388 patients were available at 6 months of follow-up for analysis of adverse effects profile. About 38.6% patients experienced adverse effects. Genitourinary tract infection was the most common adverse effect (20.6%) followed by generalized weakness (10.5%). Significant reduction in mean weight and HbA1c reduction seen at 6 months (n = 202): 3.2 kg and 1.26%, respectively, and at 12 months (n = 104): 3.9 kg and 1.27%, respectively.
Conclusion: In this real-world study of patients with T2DM living in hot climate, use of SGLT2i was associated with adverse effects in higher proportion of patients than those reported in clinical trials, but effectiveness was comparable. Patient guidance regarding adequate hydration and hygiene can maximize the benefits of this promising class of drugs.
Keywords: Effectiveness; SGLT2i; real world; safety; type 2 diabetes.
Conflict of interest statement
There are no conflicts of interest.
Figures
References
-
- Group AC, Patel A, MacMahon S, Chalmers J, Neal B, Billot L, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–72. - PubMed
-
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–53. - PubMed
-
- Foroutan N, Muratov S, Levine M. Safety and efficacy of dipeptidyl peptidase-4 inhibitors vs sulfonylurea in metformin-based combination therapy for type 2 diabetes mellitus: Systematic review and meta-analysis. Clin Invest Med. 2016;39:E48–62. - PubMed
-
- Gross JL, Kramer CK, Leitao CB, Hawkins N, Viana LV, Schaan BD, et al. Effect of antihyperglycemic agents added to metformin and a sulfonylurea on glycemic control and weight gain in type 2 diabetes: A network meta-analysis. Ann Intern Med. 2011;154:672–9. - PubMed
-
- Bakris GL, Fonseca VA, Sharma K, Wright EM. Renal sodium-glucose transport: Role in diabetes mellitus and potential clinical implications. Kidney Int. 2009;75:1272–7. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous