Potential Role of lncRNA H19 as a Cancer Biomarker in Human Cancers Detection and Diagnosis: A Pooled Analysis Based on 1585 Subjects
- PMID: 31016202
- PMCID: PMC6444267
- DOI: 10.1155/2019/9056458
Potential Role of lncRNA H19 as a Cancer Biomarker in Human Cancers Detection and Diagnosis: A Pooled Analysis Based on 1585 Subjects
Abstract
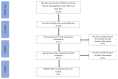
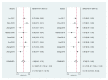
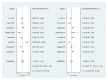
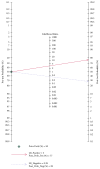
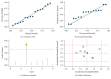
Long noncoding RNAs (lncRNAs) have been reported to serve as diagnostic and prognostic biomarkers of cancers, which play vital roles in tumorigenesis and tumor progression. Several studies have been performed to explore diagnostic value of lncRNA H19 in cancer detection and diagnosis. However, there are still inconsistent results in diagnostic accuracy and reliability in individual studies. Therefore, the present study was performed to summarize the overall diagnostic performance of lncRNA H19 in cancer detection and diagnosis. A total of eight studies with 770 cases and 815 controls were included in this pooled analysis. The pooled diagnostic results were as follows: sensitivity, 0.69 (95%CI=0.62-0.76), specificity, 0.79 (95% CI=0.70-0.86), positive likelihood ratio (PLR), 3.31 (95%CI=2.29-4.78), negative likelihood (NLR), 0.39 (95%CI=0.31-0.49), diagnostic odds ratio (DOR), 8.53 (95%CI=4.99-14.60), and area under the curve (AUC), 0.79 (95%CI=0.76-0.83). Deeks' funnel plot asymmetry test (P=0.13) suggested no potential publication bias. Our results indicated that lncRNA H19 had a relatively moderate accuracy in cancer detection and diagnosis. Further comprehensive prospective studies with large sample sizes are urgently required to validate our findings.
Figures




Similar articles
-
Long noncoding RNAs and circular RNAs as potential diagnostic biomarkers of inflammatory bowel diseases: a systematic review and meta-analysis.Front Immunol. 2024 Mar 8;15:1362437. doi: 10.3389/fimmu.2024.1362437. eCollection 2024. Front Immunol. 2024. PMID: 38524131 Free PMC article.
-
Long noncoding RNA MALAT1 acts as a potential biomarker in cancer diagnosis and detection: a meta-analysis.Biomark Med. 2019 Jan;13(1):45-54. doi: 10.2217/bmm-2018-0128. Epub 2018 Dec 18. Biomark Med. 2019. PMID: 30561226
-
Long Non-Coding RNA MALAT1 as a Detection and Diagnostic Molecular Marker in Various Human Cancers: A Pooled Analysis Based on 3255 Subjects.Onco Targets Ther. 2020 Jun 19;13:5807-5817. doi: 10.2147/OTT.S250796. eCollection 2020. Onco Targets Ther. 2020. PMID: 32606791 Free PMC article. Review.
-
Diagnostic value of HOX transcript antisense RNA for cancer detection: a meta-analysis.Biomark Med. 2020 Apr;14(5):401-411. doi: 10.2217/bmm-2019-0207. Epub 2020 Apr 9. Biomark Med. 2020. PMID: 32270696
-
The diagnostic and prognostic value of exosome-derived long non-coding RNAs in cancer patients: a meta-analysis.Clin Exp Med. 2020 Aug;20(3):339-348. doi: 10.1007/s10238-020-00638-z. Epub 2020 Jun 5. Clin Exp Med. 2020. PMID: 32504320 Review.
Cited by
-
Targeting non-coding RNA H19: A potential therapeutic approach in pulmonary diseases.Front Pharmacol. 2022 Sep 16;13:978151. doi: 10.3389/fphar.2022.978151. eCollection 2022. Front Pharmacol. 2022. PMID: 36188624 Free PMC article. Review.
-
Icariin attenuates endothelial-mesenchymal transition via H19/miR-148b-3p/ELF5 in ox-LDL-stimulated HUVECs.Mol Ther Nucleic Acids. 2020 Dec 3;23:464-475. doi: 10.1016/j.omtn.2020.11.021. eCollection 2021 Mar 5. Mol Ther Nucleic Acids. 2020. PMID: 33510936 Free PMC article.
-
Mitochondrial Dysfunction and Chronic Inflammation in Polycystic Ovary Syndrome.Int J Mol Sci. 2021 Apr 10;22(8):3923. doi: 10.3390/ijms22083923. Int J Mol Sci. 2021. PMID: 33920227 Free PMC article. Review.
-
Molecular therapies delaying cardiovascular aging: disease- or health-oriented approaches.Vasc Biol. 2020 Jan 16;2(1):R45-R58. doi: 10.1530/VB-19-0029. eCollection 2020. Vasc Biol. 2020. PMID: 32923974 Free PMC article. Review.
-
The relationship between H19 and parameters of ovarian reserve.Reprod Biol Endocrinol. 2020 May 13;18(1):46. doi: 10.1186/s12958-020-00578-z. Reprod Biol Endocrinol. 2020. PMID: 32404103 Free PMC article.
References
-
- Cree I. A., Kurbacher C. M., Lamont A., et al. A prospective randomized controlled trial of tumour chemosensitivity assay directed chemotherapy versus physician's choice in patients with recurrent platinum-resistant ovarian cancer. Anti-Cancer Drugs. 2007;18(9):1093–1101. doi: 10.1097/CAD.0b013e3281de727e. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

