Knockout of ISCA1 causes early embryonic death in rats
- PMID: 31016283
- PMCID: PMC6431120
- DOI: 10.1002/ame2.12059
Knockout of ISCA1 causes early embryonic death in rats
Abstract
Background: Iron-sulfur cluster assembly 1 (ISCA1) is an iron-sulfur (Fe/S) carrier protein that accepts Fe/S from a scaffold protein and transfers it to target proteins including the mitochondrial Fe/S containing proteins. ISCA1 is also the newly identified causal gene for multiple mitochondrial dysfunctions syndrome (MMDS). However, our knowledge about the physiological function of ISCA1 in vivo is currently limited. In this study, we generated an ISCA1 knockout rat line and analyzed the embryo development.
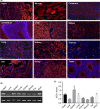
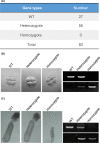
Methods: ISCA1 knockout rats were generated by replacing the exon1 of ISCA1 gene with the mCherry-Cre fusion gene using CRISPR-Cas9 technology. The ISCA1 expression pattern was analyzed by fluorescence imaging using ISCA1 promotor driven Cre and mCherry expression. The embryonic morphology was examinated by microscope and mitochondrial proteins were tested by Western blot.
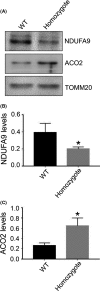
Results: An ISCA1 knockout rat line was obtained, which expressed mCherry-Cre fusion protein. Both of the fluorescence images from mCherry and Cre induced mCherry in a reporter rat strain, showing that ISCA1 expressed in most of the tissues in rats. The ISCA1 knockout resulted in abnormal development at 8.5 days, with a significant decrease of NDUFA9 protein and an increase of aconitase 2 (ACO2) in rat embryos.
Conclusion: Deletion of ISCA1 induced early death in rats. ISCA1 affected the expression of key proteins in the mitochondrial respiratory chain complex, suggesting that ISCA1 has an important influence on the respiratory complex and energy metabolism.
Keywords: ISCA1; embryonic development; energy metabolism.
Conflict of interest statement
None.
Figures

Similar articles
-
A neuron-specific Isca1 knockout rat developments multiple mitochondrial dysfunction syndromes.Animal Model Exp Med. 2023 Apr;6(2):155-167. doi: 10.1002/ame2.12318. Animal Model Exp Med. 2023. PMID: 37140997 Free PMC article.
-
Novel rat model of multiple mitochondrial dysfunction syndromes (MMDS) complicated with cardiomyopathy.Animal Model Exp Med. 2021 Dec 6;4(4):381-390. doi: 10.1002/ame2.12193. eCollection 2021 Dec. Animal Model Exp Med. 2021. PMID: 34977489 Free PMC article.
-
Myocardium-specific Isca1 knockout causes iron metabolism disorder and myocardial oncosis in rat.Life Sci. 2022 May 15;297:120485. doi: 10.1016/j.lfs.2022.120485. Epub 2022 Mar 15. Life Sci. 2022. PMID: 35304126
-
A Review of Multiple Mitochondrial Dysfunction Syndromes, Syndromes Associated with Defective Fe-S Protein Maturation.Biomedicines. 2021 Aug 10;9(8):989. doi: 10.3390/biomedicines9080989. Biomedicines. 2021. PMID: 34440194 Free PMC article. Review.
-
Novel IBA57 mutations in two chinese patients and literature review of multiple mitochondrial dysfunction syndrome.Metab Brain Dis. 2022 Feb;37(2):311-317. doi: 10.1007/s11011-021-00856-8. Epub 2021 Oct 28. Metab Brain Dis. 2022. PMID: 34709542 Review.
Cited by
-
TRDMT1 exhibited protective effects against LPS-induced inflammation in rats through TLR4-NF-κB/MAPK-TNF-α pathway.Animal Model Exp Med. 2022 Apr;5(2):172-182. doi: 10.1002/ame2.12221. Animal Model Exp Med. 2022. PMID: 35474613 Free PMC article.
-
Rat models of human diseases and related phenotypes: a systematic inventory of the causative genes.J Biomed Sci. 2020 Aug 2;27(1):84. doi: 10.1186/s12929-020-00673-8. J Biomed Sci. 2020. PMID: 32741357 Free PMC article. Review.
-
Identification of ISCA1 as novel immunological and prognostic biomarker for bladder cancer.Front Immunol. 2022 Aug 22;13:975503. doi: 10.3389/fimmu.2022.975503. eCollection 2022. Front Immunol. 2022. PMID: 36072584 Free PMC article.
-
Conditional knockdown of integrin beta-3 reveals its involvement in osteolytic and soft tissue lesions of breast cancer skeletal metastasis.J Cancer Res Clin Oncol. 2021 Feb;147(2):361-371. doi: 10.1007/s00432-020-03428-y. Epub 2020 Oct 20. J Cancer Res Clin Oncol. 2021. PMID: 33083904 Free PMC article.
-
A neuron-specific Isca1 knockout rat developments multiple mitochondrial dysfunction syndromes.Animal Model Exp Med. 2023 Apr;6(2):155-167. doi: 10.1002/ame2.12318. Animal Model Exp Med. 2023. PMID: 37140997 Free PMC article.
References
-
- Beilschmidt LK, Puccio HM. Mammalian Fe‐S cluster biogenesis and its implication in disease. Biochimie. 2014;100:48‐60. - PubMed
-
- Lill R. Function and biogenesis of iron‐sulphur proteins. Nature. 2009;460(7257):831‐838. - PubMed
-
- Huber C, Eisenreich W, Hecht S, Wachtershauser G. A possible primordial peptide cycle. Science. 2003;301(5635):938‐940. - PubMed
-
- Zeng J, Geng M, Jiang H, Liu Y, Liu J, Qiu G. The IscA from Acidithiobacillus ferrooxidans is an iron‐sulfur protein which assemble the [Fe4S4] cluster with intracellular iron and sulfur. Arch Biochem Biophys. 2007;463(2):237‐244. - PubMed
-
- Colin F, Martelli A, Clemancey M, et al. Mammalian frataxin controls sulfur production and iron entry during de novo Fe4S4 cluster assembly. J Am Chem Soc. 2013;135(2):733‐740. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

