Nerve growth factor gene therapy improves bone marrow sensory innervation and nociceptor-mediated stem cell release in a mouse model of type 1 diabetes with limb ischaemia
- PMID: 31016359
- PMCID: PMC6560027
- DOI: 10.1007/s00125-019-4860-y
Nerve growth factor gene therapy improves bone marrow sensory innervation and nociceptor-mediated stem cell release in a mouse model of type 1 diabetes with limb ischaemia
Abstract
Aims/hypothesis: Sensory neuropathy is common in people with diabetes; neuropathy can also affect the bone marrow of individuals with type 2 diabetes. However, no information exists on the state of bone marrow sensory innervation in type 1 diabetes. Sensory neurons are trophically dependent on nerve growth factor (NGF) for their survival. The aim of this investigation was twofold: (1) to determine if sensory neuropathy affects the bone marrow in a mouse model of type 1 diabetes, with consequences for stem cell liberation after tissue injury; and (2) to verify if a single systemic injection of the NGF gene exerts long-term beneficial effects on these phenomena.
Methods: A mouse model of type 1 diabetes was generated in CD1 mice by administration of streptozotocin; vehicle was administered to non-diabetic control animals. Diabetic animals were randomised to receive systemic gene therapy with either human NGF or β-galactosidase. After 13 weeks, limb ischaemia was induced in both groups to study the recovery post injury. When the animals were killed, samples of tissue and peripheral blood were taken to assess stem cell mobilisation and homing, levels of substance P and muscle vascularisation. An in vitro cellular model was adopted to verify signalling downstream to human NGF and related neurotrophic or pro-apoptotic effects. Normally distributed variables were compared between groups using the unpaired Student's t test and non-normally distributed variables were assessed by the Wilcoxon-Mann-Whitney test. The Fisher's exact test was employed for categorical variables.
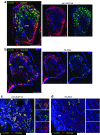
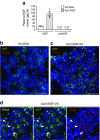
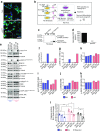
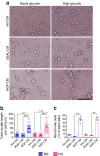
Results: Immunohistochemistry indicated a 3.3-fold reduction in the number of substance P-positive nociceptive fibres in the bone marrow of type 1 diabetic mice (p < 0.001 vs non-diabetic). Moreover, diabetes abrogated the creation of a neurokinin gradient which, in non-diabetic mice, favoured the mobilisation and homing of bone-marrow-derived stem cells expressing the substance P receptor neurokinin 1 receptor (NK1R). Pre-emptive gene therapy with NGF prevented bone marrow denervation, contrasting with the inhibitory effect of diabetes on the mobilisation of NK1R-expressing stem cells, and restored blood flow recovery from limb ischaemia. In vitro hNGF induced neurite outgrowth and exerted anti-apoptotic actions on rat PC12 cells exposed to high glucose via activation of the canonical neurotrophic tyrosine kinase receptor type 1 (TrkA) signalling pathway.
Conclusions/interpretation: This study shows, for the first time, the occurrence of sensory neuropathy in the bone marrow of type 1 diabetic mice, which translates into an altered modulation of substance P and depressed release of substance P-responsive stem cells following ischaemia. NGF therapy improves bone marrow sensory innervation, with benefits for healing on the occurrence of peripheral ischaemia. Nociceptors may represent a new target for the treatment of ischaemic complications in diabetes.
Keywords: Bone marrow; Bone marrow stem cells; Gene therapy; Nerve growth factor; Nociceptor; PC12 cells; Peripheral ischaemia; Sensory neuropathy; Substance P; Type 1 diabetes.
Figures



Similar articles
-
Sensory neuropathy hampers nociception-mediated bone marrow stem cell release in mice and patients with diabetes.Diabetologia. 2015 Nov;58(11):2653-62. doi: 10.1007/s00125-015-3735-0. Epub 2015 Sep 10. Diabetologia. 2015. PMID: 26358583 Free PMC article.
-
Nerve growth factor supplementation reverses the impairment, induced by Type 1 diabetes, of hindlimb post-ischaemic recovery in mice.Diabetologia. 2004 Jun;47(6):1055-63. doi: 10.1007/s00125-004-1424-5. Epub 2004 Jun 8. Diabetologia. 2004. PMID: 15184980
-
Nerve growth factor promotes reparative angiogenesis and inhibits endothelial apoptosis in cutaneous wounds of Type 1 diabetic mice.Diabetologia. 2004 Jun;47(6):1047-54. doi: 10.1007/s00125-004-1414-7. Epub 2004 May 26. Diabetologia. 2004. PMID: 15164170
-
Painless Nerve Growth Factor: A TrkA biased agonist mediating a broad neuroprotection via its actions on microglia cells.Pharmacol Res. 2019 Jan;139:17-25. doi: 10.1016/j.phrs.2018.10.028. Epub 2018 Nov 1. Pharmacol Res. 2019. PMID: 30391352 Review.
-
Neurotrophins and peripheral neuropathy.Philos Trans R Soc Lond B Biol Sci. 1996 Mar 29;351(1338):449-54. doi: 10.1098/rstb.1996.0041. Philos Trans R Soc Lond B Biol Sci. 1996. PMID: 8730784 Review.
Cited by
-
Moschus ameliorates glutamate-induced cellular damage by regulating autophagy and apoptosis pathway.Sci Rep. 2023 Oct 30;13(1):18586. doi: 10.1038/s41598-023-45878-7. Sci Rep. 2023. PMID: 37903904 Free PMC article.
-
Effect of 28 days treatment of baricitinib on mechanical allodynia, osteopenia, and loss of nerve fibers in an experimental model of type-1 diabetes mellitus.Pharmacol Rep. 2024 Oct;76(5):1079-1088. doi: 10.1007/s43440-024-00634-0. Epub 2024 Aug 19. Pharmacol Rep. 2024. PMID: 39155357
-
Hematopoietic Progenitors and the Bone Marrow Niche Shape the Inflammatory Response and Contribute to Chronic Disease.Int J Mol Sci. 2022 Feb 17;23(4):2234. doi: 10.3390/ijms23042234. Int J Mol Sci. 2022. PMID: 35216355 Free PMC article. Review.
-
Impaired Hematopoietic Stem/Progenitor Cell Traffic and Multi-organ Damage in Diabetes.Stem Cells. 2022 Aug 25;40(8):716-723. doi: 10.1093/stmcls/sxac035. Stem Cells. 2022. PMID: 35552468 Free PMC article.
-
Potential therapeutic effect of NK1R antagonist in diabetic non-healing wound and depression.Front Endocrinol (Lausanne). 2023 Jan 4;13:1077514. doi: 10.3389/fendo.2022.1077514. eCollection 2022. Front Endocrinol (Lausanne). 2023. PMID: 36686487 Free PMC article.
References
-
- (2008) The global challenge of diabetes. Lancet 371: 1723 - PubMed
-
- Ismail-Beigi Faramarz, Craven Timothy, Banerji Mary Ann, Basile Jan, Calles Jorge, Cohen Robert M, Cuddihy Robert, Cushman William C, Genuth Saul, Grimm Richard H, Hamilton Bruce P, Hoogwerf Byron, Karl Diane, Katz Lois, Krikorian Armand, O'Connor Patrick, Pop-Busui Rodica, Schubart Ulrich, Simmons Debra, Taylor Harris, Thomas Abraham, Weiss Daniel, Hramiak Irene. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. The Lancet. 2010;376(9739):419–430. doi: 10.1016/S0140-6736(10)60576-4. - DOI - PMC - PubMed
-
- Pop-Busui R., Evans G. W., Gerstein H. C., Fonseca V., Fleg J. L., Hoogwerf B. J., Genuth S., Grimm R. H., Corson M. A., Prineas R. Effects of Cardiac Autonomic Dysfunction on Mortality Risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Trial. Diabetes Care. 2010;33(7):1578–1584. doi: 10.2337/dc10-0125. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

