Ablation of aryl hydrocarbon receptor promotes angiotensin II-induced cardiac fibrosis through enhanced c-Jun/HIF-1α signaling
- PMID: 31016362
- PMCID: PMC7395242
- DOI: 10.1007/s00204-019-02446-1
Ablation of aryl hydrocarbon receptor promotes angiotensin II-induced cardiac fibrosis through enhanced c-Jun/HIF-1α signaling
Abstract
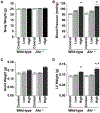
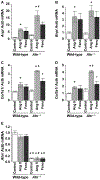
Aryl hydrocarbon receptor (AHR) is a transcription factor that binds to DNA as a heterodimer with the AHR nuclear translocator (ARNT) after interaction with ligands, such as polycyclic and halogenated aromatic hydrocarbons and other xenobiotics. The endogenous ligands and functions of AHR have been the subject of many investigations. In the present study, the potential role of AHR signaling in the development of left ventricular hypertrophy and cardiac fibrosis by angiotensin II (Ang II) infusion was investigated in mice lacking the AHR gene (Ahr-/-). We also assessed the hypothesis that fenofibrate, a peroxisome proliferator-activated receptor-α (PPARα) activator, reduces cardiac fibrosis through the c-Jun signaling. Male Ahr-/- and age-matched wild-type mice (n = 8 per group) were infused with Ang II at 100 ng/kg/min daily for 2 weeks. Treatment with Ang II increased systolic blood pressure to comparable levels in Ahr-/- and wild-type mice. However, Ahr-/- mice developed severe cardiac fibrosis after Ang II infusion compared with wild-type mice. Ang II infusion also significantly increased the expression of endothelin in the left ventricles of Ahr-/- mice, but not in wild-type mice, and significantly increased the c-Jun signaling in Ahr-/- mice. Ang II infusion also significantly enhanced the expression of hypoxia-inducible factor-1α (HIF-1α) and the downstream target vascular endothelial growth factor (VEGF) in the left ventricles of Ahr-/- mice. These results suggested pathogenic roles for the AHR signaling pathway in the development of cardiac fibrosis. Treatment with fenofibrate reduced cardiac fibrosis and abrogated the effects of Ang II on the expression of endothelin, HIF-1α, and VEGF. The inhibitory effect of fenofibrate on cardiac fibrosis was mediated by suppression of VEGF expression through modulation of c-Jun/HIF-1α signaling.
Keywords: AHR; Angiotensin II; Cardiac hypertrophy; Fibrosis; HIF-1α; PPARα; Vascular endothelial growth factor.
Conflict of interest statement
Conflict of interest The authors declare no conflict of interest.
Figures




Similar articles
-
A role for the aryl hydrocarbon receptor in regulation of ischemia-induced angiogenesis.Arterioscler Thromb Vasc Biol. 2007 Jun;27(6):1297-304. doi: 10.1161/ATVBAHA.106.138701. Epub 2007 Apr 5. Arterioscler Thromb Vasc Biol. 2007. PMID: 17413038
-
Aryl hydrocarbon receptor null mice develop cardiac hypertrophy and increased hypoxia-inducible factor-1alpha in the absence of cardiac hypoxia.Cardiovasc Toxicol. 2002;2(4):263-74. doi: 10.1385/ct:2:4:263. Cardiovasc Toxicol. 2002. PMID: 12665660
-
NcoA2-Dependent Inhibition of HIF-1α Activation Is Regulated via AhR.Toxicol Sci. 2015 Dec;148(2):517-30. doi: 10.1093/toxsci/kfv199. Epub 2015 Sep 8. Toxicol Sci. 2015. PMID: 26350169
-
Deciphering the roles of aryl hydrocarbon receptor (AHR) in regulating carcinogenesis.Toxicology. 2023 Aug 15;495:153596. doi: 10.1016/j.tox.2023.153596. Epub 2023 Jul 20. Toxicology. 2023. PMID: 37480978 Review.
-
Role of AHR and HIF-1α in Glioblastoma Metabolism.Trends Endocrinol Metab. 2017 Jun;28(6):428-436. doi: 10.1016/j.tem.2017.02.009. Epub 2017 Mar 16. Trends Endocrinol Metab. 2017. PMID: 28318896 Free PMC article. Review.
Cited by
-
Aryl Hydrocarbon Receptor Pathway Augments Peritoneal Fibrosis in a Murine CKD Model Exposed to Peritoneal Dialysate.Kidney360. 2024 Sep 1;5(9):1238-1250. doi: 10.34067/KID.0000000000000516. Epub 2024 Sep 5. Kidney360. 2024. PMID: 39235862 Free PMC article.
-
Inhibition of HIF-1α Attenuates Silica-Induced Pulmonary Fibrosis.Int J Environ Res Public Health. 2022 Jun 1;19(11):6775. doi: 10.3390/ijerph19116775. Int J Environ Res Public Health. 2022. PMID: 35682354 Free PMC article.
-
Arisaema heterophyllum Blume Monomer Stigmasterol Targets PPARγ and Inhibits the Viability and Tumorigenicity of Lung Adenocarcinoma Cells NCI-H1975.Evid Based Complement Alternat Med. 2022 Jul 20;2022:5377690. doi: 10.1155/2022/5377690. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 35911149 Free PMC article.
-
Aryl Hydrocarbon Receptor (AHR) Ligands as Selective AHR Modulators (SAhRMs).Int J Mol Sci. 2020 Sep 11;21(18):6654. doi: 10.3390/ijms21186654. Int J Mol Sci. 2020. PMID: 32932962 Free PMC article. Review.
-
Elucidating the fundamental fibrotic processes driving abdominal adhesion formation.Nat Commun. 2020 Aug 13;11(1):4061. doi: 10.1038/s41467-020-17883-1. Nat Commun. 2020. PMID: 32792541 Free PMC article.
References
-
- Balakumar P, Rohilla A, Mahadevan N (2011) Pleiotropic actions of fenofibrate on the heart. Pharmacol Res 63:8–12 - PubMed
-
- Carreira VS, Fan Y, Kurita H, Wang Q, Ko CI, Naticchioni M, Jiang M, Koch S, Zhang X, Biesiada J, Medvedovic M, Xia Y, Rubinstein J, Puga A (2015) Disruption of Ah receptor signaling during mouse development leads to abnormal cardiac structure and function in the adult. PLoS One 10:e0142440. - PMC - PubMed
-
- Duhaney TA, Cui L, Rude MK, Lebrasseur NK, Ngoy S, De Silva DS, Siwik DA, Liao R, Sam F (2007) Peroxisome proliferator-activated receptor alpha-independent actions of fenofibrate exac-erbates left ventricular dilation and fibrosis in chronic pressure overload. Hypertension 49:1084–1094 - PubMed
-
- Fernandez-Salguero P, Pineau T, Hilbert DM, McPhail T, Lee SS, Kim S, Nebert DW, Rudikoff S, Ward JM, Gonzalez FJ (1995) Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science 268:722–726 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

