Enhanced Proapoptotic Effects of Water Dispersed Complexes of 4-Thiazolidinone-Based Chemotherapeutics with a PEG-Containing Polymeric Nanocarrier
- PMID: 31016407
- PMCID: PMC6478785
- DOI: 10.1186/s11671-019-2945-7
Enhanced Proapoptotic Effects of Water Dispersed Complexes of 4-Thiazolidinone-Based Chemotherapeutics with a PEG-Containing Polymeric Nanocarrier
Abstract
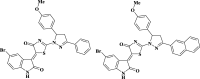
Aim: To study whether water formulation of the complex of 4-thiazolidinone derivatives with a PEG-containing polymeric nanocarrier enhances their pro-apoptotic action towards rat glioma C6 cells.
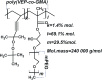
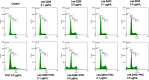
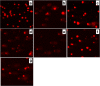
Methods: Mechanisms of antineoplastic effects of 4-thiazolidinone derivatives were investigated in vitro with rat glioma C6 cells. Cell nativity, cell cycling pattern, and Annexin V expression were evaluated and DNA damage was estimated by DNA comet analysis. A novel water-based formulation of 4-thiazolidinone derivatives complexed with a polymeric nanocarrier was utilized for enhancing pro-apoptotic action towards C6 cells.
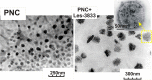
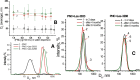
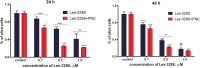
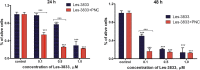
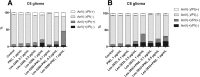
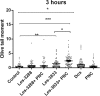
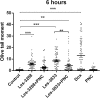
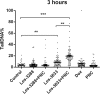
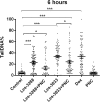
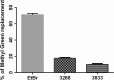
Results: The studied 4-thiazolidinone derivatives use apoptosis mechanisms for killing rat glioma C6 cells, as confirmed by FACS analysis of these cells in pre-G1 stage, the appearance of Annexin V positive C6 cells, and an increased number of DNA comets of higher classes. Complexation of the studied compounds with a PEG-containing polymeric nanocarrier significantly increased pro-apoptotic effects in rat glioma C6 cells measured by all methods mentioned above.
Conclusion: Complexation of 4-thiazolidinone derivatives with a PEG-containing polymeric nanocarrier provided them with water solubility and enhanced pro-apoptotic effects in rat glioma C6 cells.
Keywords: 4-thiazolidinones; Apoptosis; Polyethylene glycol; Polymeric nanocarrier; Rat glioma C6 cells.
Conflict of interest statement
Competing Interests
All authors have completed the Unified Competing Interest form at
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
Putative anticancer potential of novel 4-thiazolidinone derivatives: cytotoxicity toward rat C6 glioma in vitro and correlation of general toxicity with the balance of free radical oxidation in rats.Croat Med J. 2016 Apr 23;57(2):151-63. doi: 10.3325/cmj.2016.57.151. Croat Med J. 2016. PMID: 27106357 Free PMC article.
-
Multifunctional nanocarrier mediated co-delivery of doxorubicin and siRNA for synergistic enhancement of glioma apoptosis in rat.Biomaterials. 2012 Feb;33(4):1170-9. doi: 10.1016/j.biomaterials.2011.10.057. Epub 2011 Nov 5. Biomaterials. 2012. PMID: 22061491
-
Novel photodynamic therapy using water-dispersed TiO2-polyethylene glycol compound: evaluation of antitumor effect on glioma cells and spheroids in vitro.Photochem Photobiol. 2010 Jul-Aug;86(4):964-71. doi: 10.1111/j.1751-1097.2010.00742.x. Epub 2010 May 13. Photochem Photobiol. 2010. PMID: 20492566
-
Biochemical indicators of nephrotoxicity in blood serum of rats treated with novel 4-thiazolidinone derivatives or their complexes with polyethylene glycol-containing nanoscale polymeric carrier.Ukr Biochem J. 2016 Jan-Feb;88(1):51-60. doi: 10.15407/ubj88.01.051. Ukr Biochem J. 2016. PMID: 29227079
-
The Role of 4-Thiazolidinone Scaffold in Targeting Variable Biomarkers and Pathways Involving Cancer.Anticancer Agents Med Chem. 2022;22(8):1458-1477. doi: 10.2174/1871520621666210706104227. Anticancer Agents Med Chem. 2022. PMID: 34229596 Review.
Cited by
-
Recent advances in synthetic strategies and SAR of thiazolidin-4-one containing molecules in cancer therapeutics.Cancer Metastasis Rev. 2023 Sep;42(3):847-889. doi: 10.1007/s10555-023-10106-1. Epub 2023 May 19. Cancer Metastasis Rev. 2023. PMID: 37204562 Free PMC article. Review.
-
PEG-Polymeric Nanocarriers Alleviate the Immunosuppressive Effects of Free 4-Thiazolidinone-Based Chemotherapeutics on T Lymphocyte Function and Cytokine Production.Int J Nanomedicine. 2024 Dec 27;19:14021-14041. doi: 10.2147/IJN.S479137. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39742092 Free PMC article.
References
-
- Ali R, Mirza Z, Ashraf GM, Kamal MA, Ansari SA, Damanhouri GA, et al. New anticancer agents: recent developments in tumor therapy. Anticancer Res. 2012;32(7):2999–3005. - PubMed
-
- Faqi AS. A comprehensive guide to toxicology in preclinical drug development. San Diego: Elsevier; 2013. p. 885.
LinkOut - more resources
Full Text Sources
Other Literature Sources

