Effectiveness of Remission Induction Strategies for Early Rheumatoid Arthritis: a Systematic Literature Review
- PMID: 31016409
- PMCID: PMC6478774
- DOI: 10.1007/s11926-019-0821-1
Effectiveness of Remission Induction Strategies for Early Rheumatoid Arthritis: a Systematic Literature Review
Abstract
Purpose of review: To review the effectiveness of remission induction strategies compared to single csDMARD-initiating strategies according to current guidelines in early RA.
Recent findings: Twenty-nine studies, heterogeneous on, e.g., specific treatment strategy and remission outcome used, were identified. Using DAS28-remission over 12 months, 13 (76%) of 17 remission induction strategies showed significantly more patients achieving remission. Pooled relative "risk" was 1.73 [95%CI 1.59-1.88] for bDMARD-based remission induction strategies and 1.20 [95%CI 1.03-1.40] for combination csDMARD-based remission induction strategies compared to single csDMARD-initiating strategies. When additional glucocorticoid "bridging therapy" was used in single csDMARD-initiating strategies, the higher proportion patients achieving remission in remission induction strategies was no longer statistically significant (pooled RR 1.06 [95%CI 0.83-1.35]). For other remission outcomes, results were in line with above. Remission induction strategies are more effective in achieving remission compared to single csDMARD-initiating strategies, possibly more so in bDMARD-based induction strategies. However, compared to single csDMARD-initiating strategies with glucocorticoids, induction strategies may not be more effective.
Keywords: Early rheumatoid arthritis; GCs; Induction therapy; Standard care; bDMARDs; csDMARDs.
Conflict of interest statement
Dr. Lafeber reports grants from Roche, outside the submitted work.
Dr. Bijlsma reports grants from Pfizer, grants from Merck Sharp & Dohme, grants from Bristol-Myers Squibb, grants from AbbVie, grants from Roche, outside the submitted work.
Dr. van Laar reports grants from Arthrogen, grants from MSD, personal fees from Pfizer, personal fees from Eli Lilly, personal fees from BMS, grants from Astra Zeneca, grants from Roche-Genentech, outside the submitted work.
M.M.A. Verhoeven, P.M.J. Welsing, J. Tekstra and J.W.G. Jacobs declare they have no conflicts to disclose.
Figures


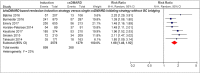

References
-
- Smolen JS, Landewé R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, Nam J, Ramiro S, Voshaar M, van Vollenhoven R, Aletaha D, Aringer M, Boers M, Buckley CD, Buttgereit F, Bykerk V, Cardiel M, Combe B, Cutolo M, van Eijk-Hustings Y, Emery P, Finckh A, Gabay C, Gomez-Reino J, Gossec L, Gottenberg JE, Hazes JMW, Huizinga T, Jani M, Karateev D, Kouloumas M, Kvien T, Li Z, Mariette X, McInnes I, Mysler E, Nash P, Pavelka K, Poór G, Richez C, van Riel P, Rubbert-Roth A, Saag K, da Silva J, Stamm T, Takeuchi T, Westhovens R, de Wit M, van der Heijde D. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76:960–977. doi: 10.1136/annrheumdis-2016-210715. - DOI - PubMed
-
- Singh JA, Saag KG, Bridges SL, et al. 2015 American College of Rheumatology Guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68:1–26. - PubMed
-
- Bijlsma JWJ, Welsing PMJ, Woodworth TG, et al. Early rheumatoid arthritis treated with tocilizumab, methotrexate, or their combination (U-Act-Early): a multicentre, randomised, double-blind, double-dummy, strategy trial. Lancet. 2016;388(10042):343–355. doi: 10.1016/S0140-6736(16)30363-4. - DOI - PubMed
-
- Dougados M, van der Heijde D, Chen Y-C, Greenwald M, Drescher E, Liu J, Beattie S, Witt S, de la Torre I, Gaich C, Rooney T, Schlichting D, de Bono S, Emery P. Baricitinib in patients with inadequate response or intolerance to conventional synthetic DMARDs: results from the RA-BUILD study. Ann Rheum Dis. 2017;76:88–95. doi: 10.1136/annrheumdis-2016-210094. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

