A Prospective Clinical Study Characterizing the Influence of Morbid Obesity on the Pharmacokinetics of Gentamicin: Towards Individualized Dosing in Obese Patients
- PMID: 31016671
- PMCID: PMC6768900
- DOI: 10.1007/s40262-019-00762-4
A Prospective Clinical Study Characterizing the Influence of Morbid Obesity on the Pharmacokinetics of Gentamicin: Towards Individualized Dosing in Obese Patients
Abstract
Background and objective: Gentamicin is an aminoglycoside antibiotic predominantly used in bloodstream infections. Although the prevalence of obesity is increasing dramatically, there is no consensus on how to adjust the dose in obese individuals. In this prospective clinical study, we study the pharmacokinetics of gentamicin in morbidly obese and non-obese individuals to develop a dosing algorithm that results in adequate drug exposure across body weights.
Methods: Morbidly obese subjects undergoing bariatric surgery and non-obese healthy volunteers received one intravenous dose of gentamicin (obese: 5 mg/kg based on lean body weight, non-obese: 5 mg/kg based on total body weight [TBW]) with subsequent 24-h sampling. All individuals had a normal renal function. Statistical analysis, modelling and Monte Carlo simulations were performed using R version 3.4.4 and NONMEM® version 7.3.
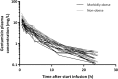
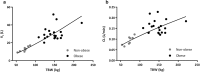
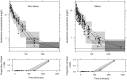
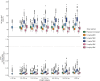
Results: A two-compartment model best described the data. TBW was the best predictor for both clearance [CL = 0.089 × (TBW/70)0.73] and central volume of distribution [Vc = 11.9 × (TBW/70)1.25] (both p < 0.001). Simulations showed how gentamicin exposure changes across the weight range with currently used dosing algorithms and illustrated that using a nomogram based on a 'dose weight' [70 × (TBW/70)0.73] will lead to similar exposure across the entire population.
Conclusions: In this study in morbidly obese and non-obese individuals ranging from 53 to 221 kg we identified body weight as an important determinant for both gentamicin CL and Vc. Using a body weight-based dosing algorithm, optimized exposure across the entire population can be achieved, thereby potentially improving efficacy and safety of gentamicin in the obese and morbidly obese population.
Trial registration: Registered in the Dutch Trial Registry (NTR6058).
Conflict of interest statement
Roger J.M. Brüggemann declares that he has no conflicts of interest with regards to this work. Outside of this work, he has served as consultant to and has received unrestricted research grants from Astellas Pharma Inc., F2G, Gilead Sciences, Merck Sharpe and Dohme Corp., and Pfizer Inc. All payments were invoiced by the Radboud University Medical Center. Cornelis Smit, Roeland E. Wasmann, Sebastiaan C. Goulooze, Eric J. Hazebroek, Eric P.A. Van Dongen, Desiree M.T. Burgers, Johan W. Mouton, and Catherijne A.J. Knibbe declare no conflicts of interest.
Figures
References
-
- EUCAST. Gentamicin: rationale for the EUCAST clinical breakpoint (version 1.2). 2009. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Rationale_do.... Accessed 2 Apr 2019.
-
- USCAST: United States Committee on Antimicrobial Susceptibility Testing. Aminoglycoside in vitro susceptibility test interpretive criteria evaluations, version 1.1. USCAST; 2016. http://www.uscast.org/. Accessed May 2018.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

