Altered vascular function in chronic kidney disease: evidence from passive leg movement
- PMID: 31016878
- PMCID: PMC6478620
- DOI: 10.14814/phy2.14075
Altered vascular function in chronic kidney disease: evidence from passive leg movement
Abstract
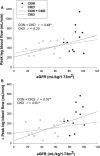
Chronic kidney disease (CKD) is an independent risk factor for the development of cardiovascular disease and is characterized by reduced nitric oxide (NO) bioavailability and vascular dysfunction, typically assessed using brachial artery flow-mediated dilation (FMD). It has been previously reported that passive leg movement (PLM)-induced hyperemia, an assessment of lower extremity vascular function, is highly dependent on NO, but has not yet been utilized to assess vascular function in patients with CKD. The purpose of this study was to comprehensively assess vascular function in patients with CKD using PLM, in addition to the traditional FMD technique. Assessment of vascular function via PLM and FMD was performed on 12 patients (CKD, 66 ± 3 years) and 16 age-matched healthy controls (CON, 60 ± 2 years). Blood velocity and artery diameters during PLM and FMD were measured using duplex ultrasound of the femoral and brachial arteries, respectively. Habitual physical activity, assessed by accelerometry, was performed in a subset of each group. CKD patients had reduced peak leg blood flow (LBF) (384 ± 39 vs. 569 ± 77 mL/min, P < 0.05) and change in LBF from baseline to peak (∆peakLBF) (143 ± 22 vs. 249 ± 34 mL/min, P < 0.05) during PLM compared to CON. Additionally, PLM responses were significantly associated with kidney function and physical activity levels. As anticipated, FMD was significantly attenuated in CKD patients (5.2 ± 1.1 vs. 8.8 ± 1.2%, P < 0.05). In conclusion, both upper and lower extremity measures of vascular function indicate impairment in CKD patients when compared to controls. PLM appears to be a novel and feasible approach to assessing lower extremity vascular function in CKD.
Keywords: CKD; PLM; endothelial function; flow-mediated dilation; nitric oxide.
© 2019 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
None declared.
Figures




Similar articles
-
Nitric oxide-mediated vascular function in sepsis using passive leg movement as a novel assessment: a cross-sectional study.J Appl Physiol (1985). 2016 May 1;120(9):991-9. doi: 10.1152/japplphysiol.00961.2015. Epub 2016 Feb 11. J Appl Physiol (1985). 2016. PMID: 26869709 Free PMC article.
-
Associations between noninvasive upper- and lower-limb vascular function assessments: extending the evidence to young women.J Appl Physiol (1985). 2022 Oct 1;133(4):886-892. doi: 10.1152/japplphysiol.00177.2022. Epub 2022 Aug 25. J Appl Physiol (1985). 2022. PMID: 36007894 Free PMC article.
-
The role of the endothelium in the hyperemic response to passive leg movement: looking beyond nitric oxide.Am J Physiol Heart Circ Physiol. 2021 Feb 1;320(2):H668-H678. doi: 10.1152/ajpheart.00784.2020. Epub 2020 Dec 11. Am J Physiol Heart Circ Physiol. 2021. PMID: 33306447 Free PMC article.
-
CORP: Ultrasound assessment of vascular function with the passive leg movement technique.J Appl Physiol (1985). 2017 Dec 1;123(6):1708-1720. doi: 10.1152/japplphysiol.00557.2017. Epub 2017 Sep 7. J Appl Physiol (1985). 2017. PMID: 28883048 Free PMC article. Review.
-
Physiological Impact and Clinical Relevance of Passive Exercise/Movement.Sports Med. 2019 Sep;49(9):1365-1381. doi: 10.1007/s40279-019-01146-1. Sports Med. 2019. PMID: 31264182 Free PMC article. Review.
Cited by
-
Endothelial dysfunction in chronic kidney disease: a clinical perspective.Am J Physiol Heart Circ Physiol. 2025 Jul 1;329(1):H135-H153. doi: 10.1152/ajpheart.00908.2024. Epub 2025 May 27. Am J Physiol Heart Circ Physiol. 2025. PMID: 40423627 Free PMC article. Review.
-
Sympathetic transduction to blood pressure in patients with chronic kidney disease.Clin Auton Res. 2025 Apr;35(2):223-230. doi: 10.1007/s10286-024-01084-7. Epub 2024 Nov 14. Clin Auton Res. 2025. PMID: 39542982
-
Vascular Health Triad in Humans With Hypertension-Not the Usual Suspects.Front Physiol. 2021 Oct 1;12:746278. doi: 10.3389/fphys.2021.746278. eCollection 2021. Front Physiol. 2021. PMID: 34658930 Free PMC article. Review.
-
The effect of exercise on vascular health in chronic kidney disease: a systematic review and meta-analysis of randomized controlled trials.Am J Physiol Renal Physiol. 2023 Nov 1;325(5):F638-F655. doi: 10.1152/ajprenal.00152.2023. Epub 2023 Sep 21. Am J Physiol Renal Physiol. 2023. PMID: 37733834 Free PMC article.
-
Physiologically Based Pharmacokinetic Modelling in Critically Ill Children Receiving Anakinra While on Extracorporeal Life Support.Clin Pharmacokinet. 2024 Sep;63(9):1343-1356. doi: 10.1007/s40262-024-01424-w. Epub 2024 Sep 27. Clin Pharmacokinet. 2024. PMID: 39331235
References
-
- Agarwal, R. 2004. Chronic kidney disease is associated with oxidative stress independent of hypertension. Clin. Nephrol. 61:377–383. - PubMed
-
- Anderson, T. J. , and Phillips S. A.. 2015. Assessment and prognosis of peripheral artery measures of vascular function. Prog. Cardiovasc. Dis. 57:497–509. - PubMed
-
- Arkouche, W. , Fouque D., Delawari E., Sibai R., Laville M., and Traeger J.. 1997. Timing of bioelectrical impedance analysis (BIA) in hemodialysis patients (HD). J. Am. Soc. Nephrol. 8:A1274. - PubMed
-
- Baylis, C. 2008. Nitric oxide deficiency in chronic kidney disease. Am. J. Physiol. Renal Physiol. 294:F1–F9. - PubMed
-
- Bonetti, P. O. , Lerman L. O., and Lerman A.. 2003. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler. Thromb. Vasc. Biol. 23:168–175. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

