Clinical features and therapeutic options in non-small cell lung cancer patients with concomitant mutations of EGFR, ALK, ROS1, KRAS or BRAF
- PMID: 31016879
- PMCID: PMC6558647
- DOI: 10.1002/cam4.2183
Clinical features and therapeutic options in non-small cell lung cancer patients with concomitant mutations of EGFR, ALK, ROS1, KRAS or BRAF
Abstract
Background: Although oncogenic driver mutations were thought to be mutually exclusive in non-small cell lung cancer (NSCLC), certain tumors harbor co-occurring mutations and represent a rare molecular subtype. The evaluation of the clinical features and therapeutic response associated with this NSCLC subtype will be vital for understanding the heterogeneity of treatment response and improving the management of these patients.
Methods: This retrospective study included 3774 samples from patients diagnosed with NSCLC. All samples were screened for EGFR, ALK, ROS1, KRAS, and BRAF mutation using the amplification-refractory mutation system. The relationship between concomitant driver mutations and clinicopathologic characteristics, and patient clinical outcomes were evaluated.
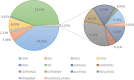
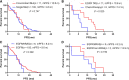
Results: Sixty-three (1.7%) samples had more than one driver gene mutation. Among these, 43 were coalterations with an EGFR mutation, 20 with an ALK rearrangement, and eight with an ROS1 rearrangement. Except for ROS1 concomitant mutations that were more frequent in male patients (87.5%, P = 0.020), the clinicopathological features of the concomitant mutation patients were not significantly different from those harboring a single EGFR, ALK, or ROS1 mutation. Furthermore, first-line EGFR-TKI treatment did not significantly improve the progression-free survival (PFS) of patients harboring EGFR concomitant mutation, compared to patients harboring a single EGFR mutation. However, for EGFR concomitant mutation patients, TKI therapy was more effective than chemotherapy (median PFS of 10.8 vs 5.2 months, P = 0.023). Lastly, KRAS mutations did not influence the EGFR-TKI therapy treatment effect.
Conclusion: In this study, concomitant mutations were found in 1.7% of the NSCLC. EGFR-TKI therapy was more effective than chemotherapy for patients harboring EGFR concomitant mutation, and ROS1 concomitant mutations were more frequent in male patients. For patients harboring coalterations with an ALK or ROS1 rearrangement, we should be cautious when considering the therapeutic options.
Keywords: ALK; EGFR; NSCLC; ROS1; concomitant mutations.
© 2019 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin‐paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947‐957. - PubMed
-
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first‐line treatment for patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (OPTIMAL, CTONG‐0802): a multicentre, open‐label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735‐742. - PubMed
-
- Solomon BJ, Mok T, Kim DW, et al. PROFILE 1014 investigators. First‐line crizotinib versus chemotherapy in ALK‐positive lung cancer. N Engl J Med. 2014;371(23):2167‐2177. - PubMed
-
- Soria JC, Ohe Y, Vansteenkiste J, et al. FLAURA investigators. Osimertinib in untreated EGFR‐Mutated advanced non‐small‐cell lung cancer. N Engl J Med. 2018;378(2):113‐125. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

