Priming Dental Pulp Stem Cells from Human Exfoliated Deciduous Teeth with Fibroblast Growth Factor-2 Enhances Mineralization Within Tissue-Engineered Constructs Implanted in Craniofacial Bone Defects
- PMID: 31016898
- PMCID: PMC6646701
- DOI: 10.1002/sctm.18-0182
Priming Dental Pulp Stem Cells from Human Exfoliated Deciduous Teeth with Fibroblast Growth Factor-2 Enhances Mineralization Within Tissue-Engineered Constructs Implanted in Craniofacial Bone Defects
Abstract
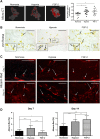
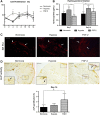
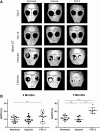
The craniofacial area is prone to trauma or pathologies often resulting in large bone damages. One potential treatment option is the grafting of a tissue-engineered construct seeded with adult mesenchymal stem cells (MSCs). The dental pulp appears as a relevant source of MSCs, as dental pulp stem cells display strong osteogenic properties and are efficient at bone formation and repair. Fibroblast growth factor-2 (FGF-2) and/or hypoxia primings were shown to boost the angiogenesis potential of dental pulp stem cells from human exfoliated deciduous teeth (SHED). Based on these findings, we hypothesized here that these primings would also improve bone formation in the context of craniofacial bone repair. We found that both hypoxic and FGF-2 primings enhanced SHED proliferation and osteogenic differentiation into plastically compressed collagen hydrogels, with a much stronger effect observed with the FGF-2 priming. After implantation in immunodeficient mice, the tissue-engineered constructs seeded with FGF-2 primed SHED mediated faster intramembranous bone formation into critical size calvarial defects than the other groups (no priming and hypoxia priming). The results of this study highlight the interest of FGF-2 priming in tissue engineering for craniofacial bone repair. Stem Cells Translational Medicine 2019;8:844&857.
Keywords: Bone engineering; Calvaria; Hypoxia; Intramembranous ossification; Mesenchymal stem cells.
© 2019 The Authors. Stem Cells Translational Medicine published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Conflict of interest statement
The authors indicated no potential conflicts of interest.
Figures




Similar articles
-
Priming Dental Pulp Stem Cells With Fibroblast Growth Factor-2 Increases Angiogenesis of Implanted Tissue-Engineered Constructs Through Hepatocyte Growth Factor and Vascular Endothelial Growth Factor Secretion.Stem Cells Transl Med. 2016 Mar;5(3):392-404. doi: 10.5966/sctm.2015-0166. Epub 2016 Jan 21. Stem Cells Transl Med. 2016. PMID: 26798059 Free PMC article.
-
Comparative characterization of stem cells from human exfoliated deciduous teeth, dental pulp, and bone marrow-derived mesenchymal stem cells.Biochem Biophys Res Commun. 2018 Jun 18;501(1):193-198. doi: 10.1016/j.bbrc.2018.04.213. Epub 2018 May 8. Biochem Biophys Res Commun. 2018. PMID: 29730288
-
Comparison of the bone regeneration ability between stem cells from human exfoliated deciduous teeth, human dental pulp stem cells and human bone marrow mesenchymal stem cells.Biochem Biophys Res Commun. 2018 Mar 11;497(3):876-882. doi: 10.1016/j.bbrc.2018.02.156. Biochem Biophys Res Commun. 2018. PMID: 29477844
-
Dental Pulp Tissue Engineering Using Mesenchymal Stem Cells: a Review with a Protocol.Stem Cell Rev Rep. 2018 Oct;14(5):668-676. doi: 10.1007/s12015-018-9826-9. Stem Cell Rev Rep. 2018. PMID: 29804171 Review.
-
A Current Overview of Scaffold-Based Bone Regeneration Strategies with Dental Stem Cells.Adv Exp Med Biol. 2020;1288:61-85. doi: 10.1007/5584_2020_505. Adv Exp Med Biol. 2020. PMID: 32185698 Review.
Cited by
-
Effect of Different Bone Grafting Materials and Mesenchymal Stem Cells on Bone Regeneration: A Micro-Computed Tomography and Histomorphometric Study in a Rabbit Calvarial Defect Model.Int J Mol Sci. 2021 Jul 28;22(15):8101. doi: 10.3390/ijms22158101. Int J Mol Sci. 2021. PMID: 34360864 Free PMC article.
-
The Journey of SCAPs (Stem Cells from Apical Papilla), from Their Native Tissue to Grafting: Impact of Oxygen Concentration.Cells. 2022 Dec 16;11(24):4098. doi: 10.3390/cells11244098. Cells. 2022. PMID: 36552862 Free PMC article.
-
Potential application of dental stem cells in regenerative reconstruction of oral and maxillofacial tissues: a narrative review.Front Oral Maxillofac Med. 2022 Jun;4:14. doi: 10.21037/fomm-21-10. Epub 2022 Jun 10. Front Oral Maxillofac Med. 2022. PMID: 35813450 Free PMC article.
-
Neurological Disease Modeling Using Pluripotent and Multipotent Stem Cells: A Key Step towards Understanding and Treating Mucopolysaccharidoses.Biomedicines. 2023 Apr 21;11(4):1234. doi: 10.3390/biomedicines11041234. Biomedicines. 2023. PMID: 37189853 Free PMC article. Review.
-
Bovine-Derived Xenografts Immobilized With Cryopreserved Stem Cells From Human Adipose and Dental Pulp Tissues Promote Bone Regeneration: A Radiographic and Histological Study.Front Bioeng Biotechnol. 2021 Apr 12;9:646690. doi: 10.3389/fbioe.2021.646690. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 33912548 Free PMC article.
References
-
- Barone A, Covani U. Maxillary alveolar ridge reconstruction with nonvascularized autogenous block bone: Clinical results. J Oral Maxillofac Surg 2007;65:2039–2046. - PubMed
-
- Nkenke E, Neukam FW. Autogenous bone harvesting and grafting in advanced jaw resorption: Morbidity, resorption and implant survival. Eur J Oral Implantol 2014;7:S203–S217. - PubMed
-
- Docquier PL, Paul L, Mousny M et al. The use of allografts in paediatric orthopaedic surgery. Acta Orthop Belg 2007;73:551–557. - PubMed
-
- Bauer TW, Muschler GF. Bone graft materials. An overview of the basic science. Clin Orthop Relat Res 2000;371:10–27. - PubMed

