Safe and Sustained Expression of Human Iduronidase After Intrathecal Administration of Adeno-Associated Virus Serotype 9 in Infant Rhesus Monkeys
- PMID: 31017018
- PMCID: PMC6703245
- DOI: 10.1089/hum.2019.012
Safe and Sustained Expression of Human Iduronidase After Intrathecal Administration of Adeno-Associated Virus Serotype 9 in Infant Rhesus Monkeys
Abstract
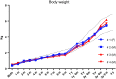
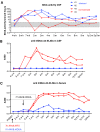
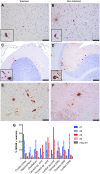
Many neuropathic diseases cause early, irreversible neurologic deterioration, which warrants therapeutic intervention during the first months of life. In the case of mucopolysaccharidosis type I, a recessive lysosomal storage disorder that results from a deficiency of the lysosomal enzyme α-l-iduronidase (IDUA), one of the most promising treatment approaches is to restore enzyme expression through gene therapy. Specifically, administering pantropic adeno-associated virus (AAV) encoding IDUA into the cerebrospinal fluid (CSF) via suboccipital administration has demonstrated remarkable efficacy in large animals. Preclinical safety studies conducted in adult nonhuman primates supported a positive risk-benefit profile of the procedure while highlighting potential subclinical toxicity to primary sensory neurons located in the dorsal root ganglia (DRG). This study investigated the long-term performance of intrathecal cervical AAV serotype 9 gene transfer of human IDUA administered to 1-month-old rhesus monkeys (N = 4) with half of the animals tolerized to the human transgene at birth via systemic administration of an AAV serotype 8 vector expressing human IDUA from the liver. Sustained expression of the transgene for almost 4 years is reported in all animals. Transduced cells were primarily pyramidal neurons in the cortex and hippocampus, Purkinje cells in the cerebellum, lower motor neurons, and DRG neurons. Both tolerized and non-tolerized animals were robust and maintained transgene expression as measured by immunohistochemical analysis of brain tissue. However, the presence of antibodies in the non-tolerized animals led to a loss of measurable levels of secreted enzyme in the CSF. These results support the safety and efficiency of treating neonatal rhesus monkeys with AAV serotype 9 gene therapy delivered into the CSF.
Keywords: AAV9; MPS I; infant; intrathecal; rhesus monkey.
Conflict of interest statement
J.M.W. is an advisor to, holds equity in, and has a sponsored research agreement with Scout Bio and Passage Bio. He also has a sponsored research agreement with Ultragenyx, Biogen, Janssen, Precision Biosciences, Moderna Therapeutics, and Amicus Therapeutics, which are licensees of Penn technology. He is an inventor on patents that have been licensed to various biopharmaceutical companies. No competing financial interests exist for the remaining authors.
Figures

Similar articles
-
Neonatal Systemic AAV Induces Tolerance to CNS Gene Therapy in MPS I Dogs and Nonhuman Primates.Mol Ther. 2015 Aug;23(8):1298-1307. doi: 10.1038/mt.2015.99. Epub 2015 May 29. Mol Ther. 2015. PMID: 26022732 Free PMC article.
-
Intrathecal administration of AAV vectors for the treatment of lysosomal storage in the brains of MPS I mice.Gene Ther. 2006 Jun;13(11):917-25. doi: 10.1038/sj.gt.3302735. Gene Ther. 2006. PMID: 16482204
-
Intranasal Adeno-Associated Virus Mediated Gene Delivery and Expression of Human Iduronidase in the Central Nervous System: A Noninvasive and Effective Approach for Prevention of Neurologic Disease in Mucopolysaccharidosis Type I.Hum Gene Ther. 2017 Jul;28(7):576-587. doi: 10.1089/hum.2017.187. Epub 2017 Apr 20. Hum Gene Ther. 2017. PMID: 28462595 Free PMC article.
-
Mucopolysaccharidoses type I gene therapy.J Inherit Metab Dis. 2021 Sep;44(5):1088-1098. doi: 10.1002/jimd.12414. Epub 2021 Jul 9. J Inherit Metab Dis. 2021. PMID: 34189746 Free PMC article. Review.
-
Comparison of high-dose intracisterna magna and lumbar puncture intrathecal delivery of AAV9 in mice to treat neuropathies.Brain Res. 2020 Jul 15;1739:146832. doi: 10.1016/j.brainres.2020.146832. Epub 2020 Apr 11. Brain Res. 2020. PMID: 32289279 Free PMC article. Review.
Cited by
-
Comparative Effectiveness of Intracerebroventricular, Intrathecal, and Intranasal Routes of AAV9 Vector Administration for Genetic Therapy of Neurologic Disease in Murine Mucopolysaccharidosis Type I.Front Mol Neurosci. 2021 May 10;14:618360. doi: 10.3389/fnmol.2021.618360. eCollection 2021. Front Mol Neurosci. 2021. PMID: 34040503 Free PMC article.
-
A Cure for Sanfilippo Syndrome? A Summary of Current Therapeutic Approaches and their Promise.Med Res Arch. 2020 Feb 1;8(2):10.18103/mra.v8i2.2045. doi: 10.18103/mra.v8i2.2045. Epub 2020 Feb 21. Med Res Arch. 2020. PMID: 32733997 Free PMC article.
-
Quantitative Whole-Body Imaging of I-124-Labeled Adeno-Associated Viral Vector Biodistribution in Nonhuman Primates.Hum Gene Ther. 2020 Dec;31(23-24):1237-1259. doi: 10.1089/hum.2020.116. Hum Gene Ther. 2020. PMID: 33233962 Free PMC article.
-
Adeno-associated virus vectors and neurotoxicity-lessons from preclinical and human studies.Gene Ther. 2025 Jan;32(1):60-73. doi: 10.1038/s41434-023-00405-1. Epub 2023 May 10. Gene Ther. 2025. PMID: 37165032 Free PMC article. Review.
-
AAVrh10 vector corrects pathology in animal models of GM1 gangliosidosis and achieves widespread distribution in the CNS of nonhuman primates.Mol Ther Methods Clin Dev. 2022 Oct 7;27:281-292. doi: 10.1016/j.omtm.2022.10.004. eCollection 2022 Dec 8. Mol Ther Methods Clin Dev. 2022. PMID: 36320411 Free PMC article.
References
-
- Vijay S, Wraith JE. Clinical presentation and follow-up of patients with the attenuated phenotype of mucopolysaccharidosis type I. Acta Paediatr 2005;94:872–877 - PubMed
-
- Matte U, Yogalingam G, Brooks D, et al. . Identification and characterization of 13 new mutations in mucopolysaccharidosis type I patients. Mol Genet Metab 2003;78:37–43 - PubMed
-
- Clarke LA, Wraith JE, Beck M, et al. . Long-term efficacy and safety of laronidase in the treatment of mucopolysaccharidosis I. Pediatrics 2009;123:229–240 - PubMed
-
- Sifuentes M, Doroshow R, Hoft R, et al. . A follow-up study of MPS I patients treated with laronidase enzyme replacement therapy for 6 years. Mol Genet Metab 2007;90:171–180 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

