Safe and Sustained Expression of Human Iduronidase After Intrathecal Administration of Adeno-Associated Virus Serotype 9 in Infant Rhesus Monkeys
- PMID: 31017018
- PMCID: PMC6703245
- DOI: 10.1089/hum.2019.012
Safe and Sustained Expression of Human Iduronidase After Intrathecal Administration of Adeno-Associated Virus Serotype 9 in Infant Rhesus Monkeys
Abstract
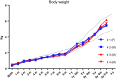
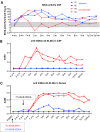
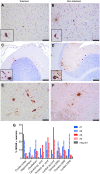
Many neuropathic diseases cause early, irreversible neurologic deterioration, which warrants therapeutic intervention during the first months of life. In the case of mucopolysaccharidosis type I, a recessive lysosomal storage disorder that results from a deficiency of the lysosomal enzyme α-l-iduronidase (IDUA), one of the most promising treatment approaches is to restore enzyme expression through gene therapy. Specifically, administering pantropic adeno-associated virus (AAV) encoding IDUA into the cerebrospinal fluid (CSF) via suboccipital administration has demonstrated remarkable efficacy in large animals. Preclinical safety studies conducted in adult nonhuman primates supported a positive risk-benefit profile of the procedure while highlighting potential subclinical toxicity to primary sensory neurons located in the dorsal root ganglia (DRG). This study investigated the long-term performance of intrathecal cervical AAV serotype 9 gene transfer of human IDUA administered to 1-month-old rhesus monkeys (N = 4) with half of the animals tolerized to the human transgene at birth via systemic administration of an AAV serotype 8 vector expressing human IDUA from the liver. Sustained expression of the transgene for almost 4 years is reported in all animals. Transduced cells were primarily pyramidal neurons in the cortex and hippocampus, Purkinje cells in the cerebellum, lower motor neurons, and DRG neurons. Both tolerized and non-tolerized animals were robust and maintained transgene expression as measured by immunohistochemical analysis of brain tissue. However, the presence of antibodies in the non-tolerized animals led to a loss of measurable levels of secreted enzyme in the CSF. These results support the safety and efficiency of treating neonatal rhesus monkeys with AAV serotype 9 gene therapy delivered into the CSF.
Keywords: AAV9; MPS I; infant; intrathecal; rhesus monkey.
Conflict of interest statement
J.M.W. is an advisor to, holds equity in, and has a sponsored research agreement with Scout Bio and Passage Bio. He also has a sponsored research agreement with Ultragenyx, Biogen, Janssen, Precision Biosciences, Moderna Therapeutics, and Amicus Therapeutics, which are licensees of Penn technology. He is an inventor on patents that have been licensed to various biopharmaceutical companies. No competing financial interests exist for the remaining authors.
Figures

References
-
- Vijay S, Wraith JE. Clinical presentation and follow-up of patients with the attenuated phenotype of mucopolysaccharidosis type I. Acta Paediatr 2005;94:872–877 - PubMed
-
- Matte U, Yogalingam G, Brooks D, et al. Identification and characterization of 13 new mutations in mucopolysaccharidosis type I patients. Mol Genet Metab 2003;78:37–43 - PubMed
-
- Clarke LA, Wraith JE, Beck M, et al. Long-term efficacy and safety of laronidase in the treatment of mucopolysaccharidosis I. Pediatrics 2009;123:229–240 - PubMed
-
- Sifuentes M, Doroshow R, Hoft R, et al. A follow-up study of MPS I patients treated with laronidase enzyme replacement therapy for 6 years. Mol Genet Metab 2007;90:171–180 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

