Modulation of proliferation and differentiation of gingiva‑derived mesenchymal stem cells by concentrated growth factors: Potential implications in tissue engineering for dental regeneration and repair
- PMID: 31017269
- PMCID: PMC6559294
- DOI: 10.3892/ijmm.2019.4172
Modulation of proliferation and differentiation of gingiva‑derived mesenchymal stem cells by concentrated growth factors: Potential implications in tissue engineering for dental regeneration and repair
Abstract
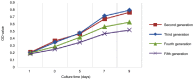
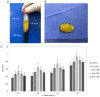
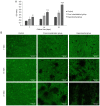
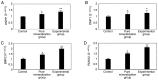
The aim of the present study was to evaluate the proliferation and osteogenic differentiation ability of gingiva‑derived mesenchymal stem cells (GMSCs) cultured with different concentrations of concentrated growth factors (CGF). GMSCs were isolated from gingival connective tissues and characterized by flow cytometry, immunofluorescence staining and immunohistochemical staining. Cell proliferation activity was determined by the MTT assay, and the effect of CGF on MCSCs was detected with the Cell Counting Kit (CCK)‑8 assay. Mineralization induction was evaluated by alkaline phosphatase (ALP)‑positive cell staining and mineralized nodule formation assay. Dentin matrix acidic phosphoprotein (DMP)1, dentin sialophosphoprotein (DSPP), bone morphogenetic protein (BMP)2 and runt‑related transcription factor (RUNX)2 mRNA and protein expression were evaluated by reverse transcription‑quantitative polymerase chain reaction (RT‑qPCR) analysis and western blotting. The flow cytometry, immunofluorescence staining and immunohistochemical staining results indicated that the cultured cells were GMSCs. The MTT assay results revealed that the third‑generation gingival stem cells exhibited the highest proliferative capacity, and the CCK‑8 results indicated that 10% CGF achieved the most prominent promotion of GMSC proliferation. ALP activity analysis and mineralized nodule assay demonstrated that CGF may successfully induce osteogenic differentiation of GMSCs, whereas RT‑qPCR and western blot analyses demonstrated that CGF is involved in the differentiation of GMSCs by regulating the expression of DMP1, DSPP, BMP2 and RUNX2 (P<0.05). In conclusion, CGF were demonstrated to promote the proliferation and osteogenic differentiation of GMSCs. Therefore, CGF may be applied in tissue engineering for tooth regeneration and repair.
Figures


Similar articles
-
[Effect of concentrated growth factor on the biological performance of human dental pulp stem cells under oxidative stress status].Zhonghua Kou Qiang Yi Xue Za Zhi. 2025 Feb 9;60(2):151-159. doi: 10.3760/cma.j.cn112144-20241021-00391. Zhonghua Kou Qiang Yi Xue Za Zhi. 2025. PMID: 39848803 Chinese.
-
Effects of enamel matrix derivative on the proliferation and osteogenic differentiation of human gingival mesenchymal stem cells.Stem Cell Res Ther. 2014 Apr 16;5(2):52. doi: 10.1186/scrt441. Stem Cell Res Ther. 2014. PMID: 24739572 Free PMC article.
-
The potential application of concentrated growth factor in pulp regeneration: an in vitro and in vivo study.Stem Cell Res Ther. 2019 May 20;10(1):134. doi: 10.1186/s13287-019-1247-4. Stem Cell Res Ther. 2019. PMID: 31109358 Free PMC article.
-
Characteristics and Tissue Regeneration Properties of Gingiva-Derived Mesenchymal Stem Cells.Crit Rev Eukaryot Gene Expr. 2015;25(2):135-44. doi: 10.1615/critreveukaryotgeneexpr.2015012539. Crit Rev Eukaryot Gene Expr. 2015. PMID: 26080607 Review.
-
Stemness Potency of Human Gingival Cells-Application in Anticancer Therapies and Clinical Trials.Cells. 2020 Aug 18;9(8):1916. doi: 10.3390/cells9081916. Cells. 2020. PMID: 32824702 Free PMC article. Review.
Cited by
-
Mechanisms of bone remodeling and therapeutic strategies in chronic apical periodontitis.Front Cell Infect Microbiol. 2022 Jul 22;12:908859. doi: 10.3389/fcimb.2022.908859. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35937695 Free PMC article. Review.
-
Effect of glucose mediated oxidative stress on apoptotic gene expression in gingival mesenchymal stem cells.BMC Oral Health. 2021 Dec 18;21(1):653. doi: 10.1186/s12903-021-02007-y. BMC Oral Health. 2021. PMID: 34922513 Free PMC article.
-
Human Oral Stem Cells, Biomaterials and Extracellular Vesicles: A Promising Tool in Bone Tissue Repair.Int J Mol Sci. 2019 Oct 9;20(20):4987. doi: 10.3390/ijms20204987. Int J Mol Sci. 2019. PMID: 31600975 Free PMC article. Review.
-
Effects of autologous concentrated growth factor on gingival thickness in periodontal accelerated osteogenic orthodontics: a 6-month randomized controlled trial.BMC Oral Health. 2021 Nov 23;21(1):604. doi: 10.1186/s12903-021-01967-5. BMC Oral Health. 2021. PMID: 34814921 Free PMC article. Clinical Trial.
-
Progress in Regenerative Medicine: Exploring Autologous Platelet Concentrates and Their Clinical Applications.Genes (Basel). 2023 Aug 23;14(9):1669. doi: 10.3390/genes14091669. Genes (Basel). 2023. PMID: 37761809 Free PMC article. Review.
References
-
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. - DOI - PubMed
-
- Tomar GB, Srivastava RK, Gupta N, Barhanpurkar AP, Pote ST, Jhaveri HM, Mishra GC, Wani MR. Human gingiva-derived mesenchymal stem cells are superior to bone marrow-derived mesenchymal stem cells for cell therapy in regenerative medicine. Biochem Biophys Res Commun. 2010;393:377–383. doi: 10.1016/j.bbrc.2010.01.126. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

