HSP90 Inhibitor, NVP-AUY922, Improves Myelination in Vitro and Supports the Maintenance of Myelinated Axons in Neuropathic Mice
- PMID: 31017387
- PMCID: PMC6588339
- DOI: 10.1021/acschemneuro.9b00105
HSP90 Inhibitor, NVP-AUY922, Improves Myelination in Vitro and Supports the Maintenance of Myelinated Axons in Neuropathic Mice
Abstract
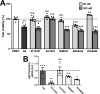
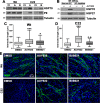
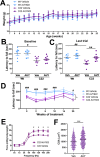
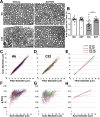
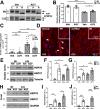
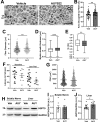
Hereditary demyelinating neuropathies linked to peripheral myelin protein 22 (PMP22) involve the disruption of normal protein trafficking and are therefore relevant targets for chaperone therapy. Using a small molecule HSP90 inhibitor, EC137, in cell culture models, we previously validated the chaperone pathway as a viable target for therapy development. Here, we tested five commercially available inhibitors of HSP90 and identified BIIB021 and AUY922 to support Schwann cell viability and enhance chaperone expression. AUY922 showed higher efficacy, compared to BIIB021, in enhancing myelin synthesis in dorsal root ganglion explant cultures from neuropathic mice. For in vivo testing, we randomly assigned 2-3 month old C22 and 6 week old Trembler J (TrJ) mice to receive two weekly injections of either vehicle or AUY922 (2 mg/kg). By the intraperitoneal (i.p.) route, the drug was well-tolerated by all mice over the 5 month long study, without influence on body weight or general grooming behavior. AUY922 improved the maintenance of myelinated nerves of both neuropathic models and attenuated the decline in rotarod performance and peak muscle force production in C22 mice. These studies highlight the significance of proteostasis in neuromuscular function and further validate the HSP90 pathway as a therapeutic target for hereditary neuropathies.
Keywords: C22 mice; Charcot-Marie-Tooth disease; Neuropathy; chaperones; myelin; neuromuscular disease.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Rapamycin activates autophagy and improves myelination in explant cultures from neuropathic mice.J Neurosci. 2010 Aug 25;30(34):11388-97. doi: 10.1523/JNEUROSCI.1356-10.2010. J Neurosci. 2010. PMID: 20739560 Free PMC article.
-
A neutral lipid-enriched diet improves myelination and alleviates peripheral nerve pathology in neuropathic mice.Exp Neurol. 2019 Nov;321:113031. doi: 10.1016/j.expneurol.2019.113031. Epub 2019 Aug 3. Exp Neurol. 2019. PMID: 31386828
-
Pharmacological induction of the heat shock response improves myelination in a neuropathic model.Neurobiol Dis. 2008 Oct;32(1):105-15. doi: 10.1016/j.nbd.2008.06.015. Epub 2008 Jul 8. Neurobiol Dis. 2008. PMID: 18655835 Free PMC article.
-
Rapamycin improves peripheral nerve myelination while it fails to benefit neuromuscular performance in neuropathic mice.Neurobiol Dis. 2014 Oct;70:224-36. doi: 10.1016/j.nbd.2014.06.023. Epub 2014 Jul 9. Neurobiol Dis. 2014. PMID: 25014022 Free PMC article.
-
Regulation of myelin-specific gene expression. Relevance to CMT1.Ann N Y Acad Sci. 1999 Sep 14;883:91-108. Ann N Y Acad Sci. 1999. PMID: 10586235 Review.
Cited by
-
Animal Models as a Tool to Design Therapeutical Strategies for CMT-like Hereditary Neuropathies.Brain Sci. 2021 Sep 18;11(9):1237. doi: 10.3390/brainsci11091237. Brain Sci. 2021. PMID: 34573256 Free PMC article. Review.
-
In Silico Drug Repurposing in Multiple Sclerosis Using scRNA-Seq Data.Int J Mol Sci. 2023 Jan 4;24(2):985. doi: 10.3390/ijms24020985. Int J Mol Sci. 2023. PMID: 36674506 Free PMC article.
-
Mechanisms and Treatments in Demyelinating CMT.Neurotherapeutics. 2021 Oct;18(4):2236-2268. doi: 10.1007/s13311-021-01145-z. Epub 2021 Nov 8. Neurotherapeutics. 2021. PMID: 34750751 Free PMC article. Review.
-
HSP70 and HSP90 in neurodegenerative diseases.Neurosci Lett. 2020 Jan 18;716:134678. doi: 10.1016/j.neulet.2019.134678. Epub 2019 Dec 6. Neurosci Lett. 2020. PMID: 31816334 Free PMC article. Review.
-
Pharmacologic Targeting of the C-Terminus of Heat Shock Protein 90 Improves Neuromuscular Function in Animal Models of Charcot Marie Tooth X1 Disease.ACS Pharmacol Transl Sci. 2023 Jan 20;6(2):306-319. doi: 10.1021/acsptsci.2c00223. eCollection 2023 Feb 10. ACS Pharmacol Transl Sci. 2023. PMID: 36798471 Free PMC article.
References
-
- Lin P. Y.; Simon S. M.; Koh W. K.; Folorunso O.; Umbaugh C. S.; Pierce A. (2013) Heat shock factor 1 over-expression protects against exposure of hydrophobic residues on mutant SOD1 and early mortality in a mouse model of amyotrophic lateral sclerosis. Mol. Neurodegener. 8, 43.10.1186/1750-1326-8-43. - DOI - PMC - PubMed
-
- Jinwal U. K.; Akoury E.; Abisambra J. F.; O’Leary J. C. 3rd; Thompson A. D.; Blair L. J.; Jin Y.; Bacon J.; Nordhues B. A.; Cockman M.; Zhang J.; Li P.; Zhang B.; Borysov S.; Uversky V. N.; Biernat J.; Mandelkow E.; Gestwicki J. E.; Zweckstetter M.; Dickey C. A. (2013) Imbalance of Hsp70 family variants fosters tau accumulation. FASEB J. 27 (4), 1450–9. 10.1096/fj.12-220889. - DOI - PMC - PubMed
-
- Mattoo R. U.; Sharma S. K.; Priya S.; Finka A.; Goloubinoff P. (2013) Hsp110 is a bona fide chaperone using ATP to unfold stable misfolded polypeptides and reciprocally collaborate with Hsp70 to solubilize protein aggregates. J. Biol. Chem. 288 (29), 21399–411. 10.1074/jbc.M113.479253. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

