Association of PDGFRB Mutations With Pediatric Myofibroma and Myofibromatosis
- PMID: 31017643
- PMCID: PMC6487901
- DOI: 10.1001/jamadermatol.2019.0114
Association of PDGFRB Mutations With Pediatric Myofibroma and Myofibromatosis
Abstract
Importance: Myofibroma is the most frequent fibrous tumor in children. Multicentric myofibroma (referred to as infantile myofibromatosis) is a life-threatening disease.
Objective: To determine the frequency, spectrum, and clinical implications of mutations in the PDGFRB receptor tyrosine kinase found in sporadic myofibroma and myofibromatosis.
Design, setting, and participants: In this retrospective study of 69 patients with sporadic myofibroma or myofibromatosis, 85 tumor samples were obtained and analyzed by targeted deep sequencing of PDGFRB. Mutations were confirmed by an alternative method of sequencing and were experimentally characterized to confirm gain of function and sensitivity to the tyrosine kinase inhibitor imatinib.
Main outcomes and measures: Frequency of gain-of-function PDGFRB mutations in sporadic myofibroma and myofibromatosis. Sensitivity to imatinib, as assessed experimentally.
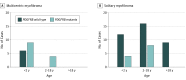
Results: Of the 69 patients with tumor samples (mean [SD] age, 7.8 [12.7] years), 60 were children (87%; 29 girls [48%]) and 9 were adults (13%; 4 women [44%]). Gain-of-function PDGFRB mutations were found in samples from 25 children, with no mutation found in samples from adults. Mutations were particularly associated with severe multicentric disease (13 of 19 myofibromatosis cases [68%]). Although patients had no familial history, 3 of 25 mutations (12%) were likely to be germline, suggesting de novo heritable alterations. All of the PDGFRB mutations were associated with ligand-independent receptor activation, and all but one were sensitive to imatinib at clinically relevant concentrations.
Conclusions and relevance: Gain-of-function mutations of PDGFRB in myofibromas may affect only children and be more frequent in the multicentric form of disease, albeit present in solitary pediatric myofibromas. These alterations may be sensitive to tyrosine kinase inhibitors. The PDGFRB sequencing appears to have a high value for diagnosis, prognosis, and therapy of soft-tissue tumors in children.
Conflict of interest statement
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous

