National Cancer Institute (NCI) state of the science: Targeted radiosensitizers in colorectal cancer
- PMID: 31017664
- PMCID: PMC6663584
- DOI: 10.1002/cncr.32150
National Cancer Institute (NCI) state of the science: Targeted radiosensitizers in colorectal cancer
Abstract
Colorectal cancer (CRC) represents a major public health problem as the second leading cause of cancer-related mortality in the United States. Of an estimated 140,000 newly diagnosed CRC cases in 2018, roughly one-third of these patients will have a primary tumor located in the distal large bowel or rectum. The current standard-of-care approach includes curative-intent surgery, often after preoperative (neoadjuvant) radiotherapy (RT), to increase rates of tumor down-staging, clinical and pathologic response, as well as improving surgical resection quality. However, despite advancements in surgical techniques, as well as sharpened precision of dosimetry offered by contemporary RT delivery platforms, the oncology community continues to face challenges related to disease relapse. Ongoing investigations are aimed at testing novel radiosensitizing agents and treatments that might exploit the systemic antitumor effects of RT using immunotherapies. If successful, these treatments may usher in a new curative paradigm for rectal cancers, such that surgical interventions may be avoided. Importantly, this disease offers an opportunity to correlate matched paired biopsies, radiographic response, and molecular mechanisms of treatment sensitivity and resistance with clinical outcomes. Herein, the authors highlight the available evidence from preclinical models and early-phase studies, with an emphasis on promising developmental therapeutics undergoing prospective validation in larger scale clinical trials. This review by the National Cancer Institute's Radiation Research Program Colorectal Cancer Working Group provides an updated, comprehensive examination of the continuously evolving state of the science regarding radiosensitizer drug development in the curative treatment of CRC.
Keywords: abscopal effect; chemoradiotherapy; colorectal cancer; immunotherapy; precision radiation medicine; radiation biology; radiation therapy; radiosensitization; rectal cancer; targeted therapeutics.
© 2019 American Cancer Society.
Conflict of interest statement
Author financial disclosures and conflicts of interest:
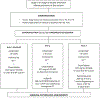
Figures


References
-
- Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA: a cancer journal for clinicians 2017. - PubMed
-
- Franke AJ, Parekh H, Starr JS, et al. Total neoadjuvant therapy: a shifting paradigm in locally advanced rectal cancer management. Clinical colorectal cancer 2018;17(1):1–12. - PubMed
-
- Steel GG, Peckham MJ. Exploitable mechanisms in combined radiotherapy-chemotherapy: the concept of additivity. Int J Radiat Oncol Biol Phys 1979;5(1):85–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

