Correlative SICM-FCM reveals changes in morphology and kinetics of endocytic pits induced by disease-associated mutations in dynamin
- PMID: 31017801
- PMCID: PMC6593877
- DOI: 10.1096/fj.201802635R
Correlative SICM-FCM reveals changes in morphology and kinetics of endocytic pits induced by disease-associated mutations in dynamin
Abstract
Dynamin 2 (DNM2) is a GTP-binding protein that controls endocytic vesicle scission and defines a whole class of dynamin-dependent endocytosis, including clathrin-mediated endocytosis by caveoli. It has been suggested that mutations in the DNM2 gene, associated with 3 inherited diseases, disrupt endocytosis. However, how exactly mutations affect the nanoscale morphology of endocytic machinery has never been studied. In this paper, we used live correlative scanning ion conductance microscopy (SICM) and fluorescence confocal microscopy (FCM) to study how disease-associated mutations affect the morphology and kinetics of clathrin-coated pits (CCPs) by directly following their dynamics of formation, maturation, and internalization in skin fibroblasts from patients with centronuclear myopathy (CNM) and in Cos-7 cells expressing corresponding dynamin mutants. Using SICM-FCM, which we have developed, we show how p.R465W mutation disrupts pit structure, preventing its maturation and internalization, and significantly increases the lifetime of CCPs. Differently, p.R522H slows down the formation of CCPs without affecting their internalization. We also found that CNM mutations in DNM2 affect the distribution of caveoli and reduce dorsal ruffling in human skin fibroblasts. Collectively, our SICM-FCM findings at single CCP level, backed up by electron microscopy data, argue for the impairment of several forms of endocytosis in DNM2-linked CNM.-Ali, T., Bednarska, J., Vassilopoulos, S., Tran, M., Diakonov, I. A., Ziyadeh-Isleem, A., Guicheney, P., Gorelik, J., Korchev, Y. E., Reilly, M. M., Bitoun, M., Shevchuk, A. Correlative SICM-FCM reveals changes in morphology and kinetics of endocytic pits induced by disease-associated mutations in dynamin.
Keywords: Charcot-Marie-Tooth; caveolin; clathrin; myopathy.
Conflict of interest statement
The authors thank Dr. P. Novak (Queen Mary University of London) for scanning software development, Dr. Andy Rogers (Royal Brompton and Harefield NHS Trust) for expertise in TEM, and Dr. Benedict Reilly-O’Donnell (National Heart and Lung Institute, Department of Cardiac Medicine, Imperial College London, London, United Kingdom) for proofreading of the manuscript. This work was supported by PhD studentship to T.A. from Muscular Dystrophy UK (Grant P49914 to A.S.), Biotechnology and Biological Sciences Research Council (BBSRC) funding to A.S. (BB/M022080/1), the Agence Nationale de la Recherche (Grant ANR-14-CE12-0009 to M.B. and Young Researcher Grant ANR-14-CE12-0001-01 to S.V.), and British Heart Foundation (BHF) funding for J.G. and I.A.D. (RG/17/13/33173). A.S. and Y.K. are shareholders in ICAPPIC, Ltd., a company commercializing nanopipette-based instrumentation. The authors declare no other conflicts of interest.
Figures

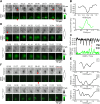
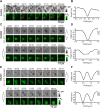


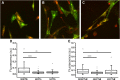

References
-
- Praefcke G. J. K., McMahon H. T. (2004) The dynamin superfamily: universal membrane tubulation and fission molecules? Nat. Rev. Mol. Cell Biol. 5, 133–147 - PubMed
-
- Bitoun M., Maugenre S., Jeannet P.-Y., Lacène E., Ferrer X., Laforêt P., Martin J.-J., Laporte J., Lochmüller H., Beggs A. H., Fardeau M., Eymard B., Romero N. B., Guicheney P. (2005) Mutations in dynamin 2 cause dominant centronuclear myopathy. Nat. Genet. 37, 1207–1209 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

