Wnt3a involved in the mechanical loading on improvement of bone remodeling and angiogenesis in a postmenopausal osteoporosis mouse model
- PMID: 31017804
- PMCID: PMC9272758
- DOI: 10.1096/fj.201802711R
Wnt3a involved in the mechanical loading on improvement of bone remodeling and angiogenesis in a postmenopausal osteoporosis mouse model
Abstract
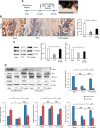
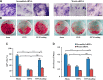
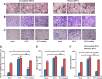
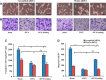
Osteoporosis is a major health problem, making bones fragile and susceptible to fracture. Previous works showed that mechanical loading stimulated bone formation and accelerated fracture healing. Focusing on the role of Wnt3a (wingless/integrated 3a), this study was aimed to assess effects of mechanical loading to the spine, using ovariectomized (OVX) mice as a model of osteoporosis. Two-week daily application of this novel loading (4 N, 10 Hz, 5 min/d) altered bone remodeling with an increase in Wnt3a. Spinal loading promoted osteoblast differentiation, endothelial progenitor cell migration, and tube formation and inhibited osteoclast formation, migration, and adhesion. A transient silencing of Wnt3a altered the observed loading effects. Spinal loading significantly increased bone mineral density, bone mineral content, and bone area per tissue area. The loaded OVX group showed a significant increase in the number of osteoblasts and reduction in osteoclast surface/bone surface. Though expression of osteoblastic genes was increased, the levels of osteoclastic genes were decreased by loading. Spinal loading elevated a microvascular volume as well as VEGF expression. Collectively, this study supports the notion that Wnt3a-mediated signaling involves in the effect of spinal loading on stimulating bone formation, inhibiting bone resorption, and promoting angiogenesis in OVX mice. It also suggests that Wnt3a might be a potential therapeutic target for osteoporosis treatment.-Li, X., Liu, D., Li, J., Yang, S., Xu, J., Yokota, H., Zhang, P. Wnt3a involved in the mechanical loading on improvement of bone remodeling and angiogenesis in a postmenopausal osteoporosis mouse model.
Keywords: OVX; bone formation; bone resorption; neovascularization; spinal load.
Figures

References
-
- Deng, L. , Wang, Y. , Peng, Y. , Wu, Y. , Ding, Y. , Jiang, Y. , Shen, Z. , and Fu, Q. (2015) Osteoblast‐derived microvesicles: a novel mechanism for communication between osteoblasts and osteoclasts. Bone 79, 37–42 - PubMed
-
- Xu, B. , Lovre, D. , and Mauvais‐Jarvis, F. (2016) Effect of selective estrogen receptor modulators on metabolic homeostasis. Biochimie 124, 92–97 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

