Formulation and Characterization of Conjugate Vaccines to Reduce Opioid Use Disorders Suitable for Pharmaceutical Manufacturing and Clinical Evaluation
- PMID: 31018096
- PMCID: PMC6598681
- DOI: 10.1021/acs.molpharmaceut.8b01296
Formulation and Characterization of Conjugate Vaccines to Reduce Opioid Use Disorders Suitable for Pharmaceutical Manufacturing and Clinical Evaluation
Abstract
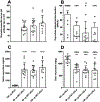
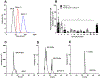
This study focused on formulating conjugate vaccines targeting oxycodone and heroin for technology transfer, good manufacturing practice (GMP), and clinical evaluation. Lead vaccines used the highly immunogenic carrier protein keyhole limpet hemocyanin (KLH), which poses formulation problems because of its size. To address this barrier to translation, an oxycodone-based hapten conjugated to GMP-grade subunit KLH (OXY-sKLH) and adsorbed on alum adjuvant was studied with regard to carbodiimide coupling reaction time, buffer composition, purification methods for conjugates, conjugate size, state of aggregation, and protein/alum ratio. Vaccine formulations were screened for post-immunization antibody levels and efficacy in reducing oxycodone distribution to the brain in rats. While larger conjugates were more immunogenic, their size prevented characterization of the haptenation ratio by standard analytical methods and sterilization by filtration. To address this issue, conjugation chemistry and vaccine formulation were optimized for maximal efficacy, and conjugate size was measured by dynamic light scattering prior to adsorption to alum. An analogous heroin vaccine (M-sKLH) was also optimized for conjugation chemistry, formulated in alum, and characterized for potency against heroin in rats. Finally, this study found that the efficacy of OXY-sKLH was preserved when co-administered with M-sKLH, supporting the proof of concept for a bivalent vaccine formulation targeting both heroin and oxycodone. This study suggests methods for addressing the unique formulation and characterization challenges posed by conjugating small molecules to sKLH while preserving vaccine efficacy.
Keywords: FDA; GLP; GMP; antibody; conjugate; heroin; opioid use disorder; oxycodone; vaccine.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


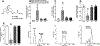
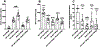
References
-
- Overdose Deaths Involving Opioids, Cocaine, and Psychostimulants - United States. https://www.cdc.gov/mmwr/volumes/67/wr/mm6712a1.htm?s_cid=mm6712a1_w, 2015–2016. (accessed 2018). - PMC - PubMed
-
- Rudd RA; Seth P; David F; Scholl L Increases in Drug and Opioid-Involved Overdose Deaths - United States, 2010–2015. MMWR Morb. Mortal. Wkly. Rep 2016, 65, 1445–1452. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

