Impact of Diagnosis and Therapy on Cognitive Function in Urea Cycle Disorders
- PMID: 31018246
- PMCID: PMC6692656
- DOI: 10.1002/ana.25492
Impact of Diagnosis and Therapy on Cognitive Function in Urea Cycle Disorders
Abstract
Objective: Individuals with urea cycle disorders (UCDs) often present with intellectual and developmental disabilities. The major aim of this study was to evaluate the impact of diagnostic and therapeutic interventions on cognitive outcomes in UCDs.
Methods: This prospective, observational, multicenter study includes data from 503 individuals with UCDs who had comprehensive neurocognitive testing with a cumulative follow-up of 702 patient-years.
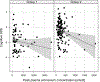
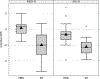
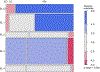
Results: The mean cognitive standard deviation score (cSDS) was lower in symptomatic than in asymptomatic (p < 0.001, t test) individuals with UCDs. Intellectual disability (intellectual quotient < 70, cSDS < -2.0) was associated with the respective subtype of UCD and early disease onset, whereas height of the initial peak plasma ammonium concentration was inversely associated with neurocognitive outcomes in mitochondrial (proximal) rather than cytosolic (distal) UCDs. In ornithine transcarbamylase and argininosuccinate synthetase 1 deficiencies, we did not find evidence that monoscavenger therapy with sodium or glycerol phenylbutyrate was superior to sodium benzoate in providing cognitive protection. Early liver transplantation appears to be beneficial for UCDs. It is noteworthy that individuals with argininosuccinate synthetase 1 and argininosuccinate lyase deficiencies identified by newborn screening had better neurocognitive outcomes than those diagnosed after the manifestation of first symptoms.
Interpretation: Cognitive function is related to interventional and non-interventional variables. Early detection by newborn screening and early liver transplantation appear to offer greater cognitive protection, but none of the currently used nitrogen scavengers was superior with regard to long-term neurocognitive outcome. Further confirmation could determine these variables as important clinical indicators of neuroprotection for individuals with UCDs. ANN NEUROL 2019.
© 2019 American Neurological Association.
Conflict of interest statement
Potential Conflicts of Interest
All other authors declare that they have nothing to report.
Figures


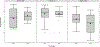
References
-
- Kido J, Nakamura K, Mitsubuchi H, et al. Long-term outcome and intervention of urea cycle disorders in Japan. J Inherit Metab Dis. 2012. September;35(5):777–85. - PubMed
-
- Burgard P, Kolker S, Haege G, Lindner M, Hoffmann GF. Neonatal mortality and outcome at the end of the first year of life in early onset urea cycle disorders--review and meta-analysis of observational studies published over more than 35 years. J Inherit Metab Dis. 2016. March;39(2):219–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U54HD090257/NH/NIH HHS/United States
- 2010 12 01/European Union/International
- Physician-Scientist Program at University of Heidelberg/International
- National Urea Cycle Disorders Foundation/International
- Dietmar Hopp Foundation/International
- National Center for Advancing Translational Science/International
- U54 HD061221/HD/NICHD NIH HHS/United States
- Rotenberg Family Fund/International
- radiz-Rare Disease Initiative Zurich/International
- EAHC no 2010 12 01/European Union/International
- Kettering Fund/International
- Eunice Kennedy Shriver National Institute of Child Health and Human Development/International
- U54 HD086984/HD/NICHD NIH HHS/United States
- Heidelberg Research Center for Molecular Medicine (HRCMM) in the framework of the Excellence Initiative II of the German Research Foundation/International
- Office of Rare Diseases Research/International
- U2C TR002818/TR/NCATS NIH HHS/United States
- O'Malley Foundation/International
- Kindness-for-Kids Foundation/International
- U54 HD090257/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

