Chronic Treatment with Multi-Kinase Inhibitors Causes Differential Toxicities on Skeletal and Cardiac Muscles
- PMID: 31018508
- PMCID: PMC6520777
- DOI: 10.3390/cancers11040571
Chronic Treatment with Multi-Kinase Inhibitors Causes Differential Toxicities on Skeletal and Cardiac Muscles
Abstract
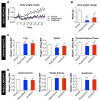
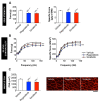
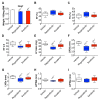
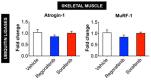
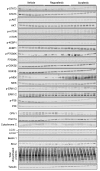
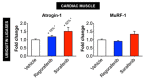
Despite recent progress, chemotherapy remains the preferred treatment for cancer. We have shown a link between anticancer drugs and the development of cachexia, i.e., body wasting accompanied by muscle loss. The multi-kinase inhibitors (MKIs) regorafenib and sorafenib, used as second-line treatment for solid tumors, are frequently accompanied by several side effects, including loss of muscle mass and strength. In the present study we aimed to investigate the molecular mechanisms associated with the occurrence of muscle toxicities in in vivo conditions. Hence, we treated 8-week old healthy CD2F1 male mice with MKIs for up to six weeks and observed decreased skeletal and cardiac muscle mass, consistent with muscle weakness. Modulation of ERK1/2 and GSK3β, as well as increased expression of markers of autophagy, previously associated with muscle atrophy conditions, were shown in skeletal muscle upon treatment with either drug. MKIs also promoted cardiac abnormalities consistent with reduced left ventricular mass, internal diameter, posterior wall thickness and stroke volume, despite unchanged overall function. Notably, different signaling pathways were affected in the heart, including reduced expression of mitochondrial proteins, and elevated AKT, GSK3β, mTOR, MEK1/2 and ERK1/2 phosphorylation. Combined, our data demonstrate detrimental effects on skeletal and cardiac muscle in association with chronic administration of MKIs, although different mechanisms would seem to contribute to the cachectic phenotype in the two tissues.
Keywords: cardiac cachexia; chemotherapy; regorafenib; skeletal muscle wasting; sorafenib.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Chemotherapy-related cachexia is associated with mitochondrial depletion and the activation of ERK1/2 and p38 MAPKs.Oncotarget. 2016 Jul 12;7(28):43442-43460. doi: 10.18632/oncotarget.9779. Oncotarget. 2016. PMID: 27259276 Free PMC article.
-
Effects of the beta2 agonist formoterol on atrophy signaling, autophagy, and muscle phenotype in respiratory and limb muscles of rats with cancer-induced cachexia.Biochimie. 2018 Jun;149:79-91. doi: 10.1016/j.biochi.2018.04.009. Epub 2018 Apr 12. Biochimie. 2018. PMID: 29654866
-
Inhibition of the renin-angiotensin system improves physiological outcomes in mice with mild or severe cancer cachexia.Int J Cancer. 2013 Sep 1;133(5):1234-46. doi: 10.1002/ijc.28128. Epub 2013 Mar 16. Int J Cancer. 2013. PMID: 23436228
-
Pathophysiology of peripheral muscle wasting in cardiac cachexia.Curr Opin Clin Nutr Metab Care. 2005 May;8(3):249-54. doi: 10.1097/01.mco.0000165002.08955.5b. Curr Opin Clin Nutr Metab Care. 2005. PMID: 15809526 Review.
-
Cardiac cachexia: a syndrome with impaired survival and immune and neuroendocrine activation.Chest. 1999 Mar;115(3):836-47. doi: 10.1378/chest.115.3.836. Chest. 1999. PMID: 10084500 Review.
Cited by
-
Impact of Sarcopenia on the Survival of Patients with Hepatocellular Carcinoma Treated with Sorafenib.Cancers (Basel). 2024 Mar 7;16(6):1080. doi: 10.3390/cancers16061080. Cancers (Basel). 2024. PMID: 38539416 Free PMC article.
-
ACVR2B antagonism as a countermeasure to multi-organ perturbations in metastatic colorectal cancer cachexia.J Cachexia Sarcopenia Muscle. 2020 Dec;11(6):1779-1798. doi: 10.1002/jcsm.12642. Epub 2020 Nov 16. J Cachexia Sarcopenia Muscle. 2020. PMID: 33200567 Free PMC article.
-
Chemotherapy-Induced Myopathy: The Dark Side of the Cachexia Sphere.Cancers (Basel). 2021 Jul 19;13(14):3615. doi: 10.3390/cancers13143615. Cancers (Basel). 2021. PMID: 34298829 Free PMC article. Review.
-
Weight and skeletal muscle loss with cabozantinib in metastatic renal cell carcinoma.J Cachexia Sarcopenia Muscle. 2022 Oct;13(5):2405-2416. doi: 10.1002/jcsm.13021. Epub 2022 Jul 28. J Cachexia Sarcopenia Muscle. 2022. PMID: 35903892 Free PMC article.
-
Effects of Voluntary Wheel Running Exercise on Chemotherapy-Impaired Cognitive and Motor Performance in Mice.Int J Environ Res Public Health. 2023 Apr 3;20(7):5371. doi: 10.3390/ijerph20075371. Int J Environ Res Public Health. 2023. PMID: 37047984 Free PMC article.
References
-
- Dewys W.D., Begg C., Lavin P.T., Band P.R., Bennett J.M., Bertino J.R., Cohen M.H., Douglass H.O., Jr., Engstrom P.F., Ezdinli E.Z., et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am. J. Med. 1980;69:491–497. doi: 10.1016/S0149-2918(05)80001-3. - DOI - PubMed
-
- Ohnuma T., Adigun R. Cancer, Anorexia and Cachexia. StatPearls; Treasure Island, FL, USA: 2018.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

