The Hippo Pathway in Prostate Cancer
- PMID: 31018586
- PMCID: PMC6523349
- DOI: 10.3390/cells8040370
The Hippo Pathway in Prostate Cancer
Abstract
Despite recent efforts, prostate cancer (PCa) remains one of the most common cancers in men. Currently, there is no effective treatment for castration-resistant prostate cancer (CRPC). There is, therefore, an urgent need to identify new therapeutic targets. The Hippo pathway and its downstream effectors-the transcriptional co-activators, Yes-associated protein (YAP) and its paralog, transcriptional co-activator with PDZ-binding motif (TAZ)-are foremost regulators of stem cells and cancer biology. Defective Hippo pathway signaling and YAP/TAZ hyperactivation are common across various cancers. Here, we draw on insights learned from other types of cancers and review the latest advances linking the Hippo pathway and YAP/TAZ to PCa onset and progression. We examine the regulatory interaction between Hippo-YAP/TAZ and the androgen receptor (AR), as main regulators of PCa development, and how uncontrolled expression of YAP/TAZ drives castration resistance by inducing cellular stemness. Finally, we survey the potential therapeutic targeting of the Hippo pathway and YAP/TAZ to overcome PCa.
Keywords: YAP/TAZ; castration resistance; feedback loops; hippo pathway; prostate cancer; signal cross-talk.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the topics analyzed, in the writing of the manuscript, or in the decision to publish.
Figures
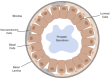
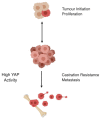



References
-
- Coleman W.B. Molecular Pathogenesis of Prostate Cancer. In: Coleman W., Tsongalis G., editors. Molecular Pathology. Elsevier; Berlin/Heidelberg, Germany: 2018. pp. 555–568.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

