Pigment Nephropathy: Novel Insights into Inflammasome-Mediated Pathogenesis
- PMID: 31018590
- PMCID: PMC6514712
- DOI: 10.3390/ijms20081997
Pigment Nephropathy: Novel Insights into Inflammasome-Mediated Pathogenesis
Abstract
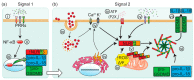
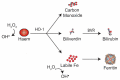
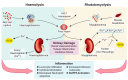
Pigment nephropathy is an acute decline in renal function following the deposition of endogenous haem-containing proteins in the kidneys. Haem pigments such as myoglobin and haemoglobin are filtered by glomeruli and absorbed by the proximal tubules. They cause renal vasoconstriction, tubular obstruction, increased oxidative stress and inflammation. Haem is associated with inflammation in sterile and infectious conditions, contributing to the pathogenesis of many disorders such as rhabdomyolysis and haemolytic diseases. In fact, haem appears to be a signalling molecule that is able to activate the inflammasome pathway. Recent studies highlight a pathogenic function for haem in triggering inflammatory responses through the activation of the nucleotide-binding domain-like receptor protein 3 (NLRP3) inflammasome. Among the inflammasome multiprotein complexes, the NLRP3 inflammasome has been the most widely characterized as a trigger of inflammatory caspases and the maturation of interleukin-18 and -1β. In the present review, we discuss the latest evidence on the importance of inflammasome-mediated inflammation in pigment nephropathy. Finally, we highlight the potential role of inflammasome inhibitors in the prophylaxis and treatment of pigment nephropathy.
Keywords: NLRP3 inflammasome; acute kidney injury; haem; pigment nephropathy; rhabdomyolysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Roles of the NLRP3 inflammasome in the pathogenesis of diabetic nephropathy.Pharmacol Res. 2016 Dec;114:251-264. doi: 10.1016/j.phrs.2016.11.004. Epub 2016 Nov 5. Pharmacol Res. 2016. PMID: 27826011 Review.
-
Effects of antidiabetic drugs on NLRP3 inflammasome activity, with a focus on diabetic kidneys.Drug Discov Today. 2019 Jan;24(1):256-262. doi: 10.1016/j.drudis.2018.08.005. Epub 2018 Aug 4. Drug Discov Today. 2019. PMID: 30086405 Review.
-
The NLRP3 inflammasome in kidney disease and autoimmunity.Nephrology (Carlton). 2016 Sep;21(9):736-44. doi: 10.1111/nep.12785. Nephrology (Carlton). 2016. PMID: 27011059 Review.
-
The purinergic 2X7 receptor participates in renal inflammation and injury induced by high-fat diet: possible role of NLRP3 inflammasome activation.J Pathol. 2013 Nov;231(3):342-53. doi: 10.1002/path.4237. Epub 2013 Sep 3. J Pathol. 2013. PMID: 23843215
-
Role of the thioredoxin interacting protein in diabetic nephropathy and the mechanism of regulating NOD‑like receptor protein 3 inflammatory corpuscle.Int J Mol Med. 2019 Jun;43(6):2440-2450. doi: 10.3892/ijmm.2019.4163. Epub 2019 Apr 11. Int J Mol Med. 2019. PMID: 31017263 Free PMC article.
Cited by
-
Interleukin-10 attenuates renal injury after myocardial infarction in diabetes.J Investig Med. 2022 Jun;70(5):1233-1242. doi: 10.1136/jim-2021-002008. Epub 2022 Feb 9. J Investig Med. 2022. PMID: 35140126 Free PMC article.
-
Microaxial Flow Pump Use and Renal Outcomes in Infarct-Related Cardiogenic Shock: A Secondary Analysis of the DanGer Shock Trial.Circulation. 2024 Dec 17;150(25):1990-2003. doi: 10.1161/CIRCULATIONAHA.124.072370. Epub 2024 Oct 27. Circulation. 2024. PMID: 39462276 Free PMC article. Clinical Trial.
-
Retrospective Evaluation of the Severity of Creatine Kinase Elevation in Canine and Feline Trauma Patients as a Predictor of Morbidity and Mortality.Vet Med (Auckl). 2025 Jul 31;16:17-23. doi: 10.2147/VMRR.S517141. eCollection 2025. Vet Med (Auckl). 2025. PMID: 40765574 Free PMC article.
-
Heme-Pigment Induced Acute Kidney Injury after Cavernous Hemangioma Ablation.Semin Intervent Radiol. 2019 Aug;36(3):275-278. doi: 10.1055/s-0039-1694064. Epub 2019 Aug 19. Semin Intervent Radiol. 2019. PMID: 31435136 Free PMC article. Review. No abstract available.
-
Nanodrugs alleviate acute kidney injury: Manipulate RONS at kidney.Bioact Mater. 2022 Sep 29;22:141-167. doi: 10.1016/j.bioactmat.2022.09.021. eCollection 2023 Apr. Bioact Mater. 2022. PMID: 36203963 Free PMC article. Review.
References
-
- Sikorski Z.E. Chemical and Functional Properties of Food Components. CRC Press; Boc Raton, FL, USA: 2007.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

