Dual-Specificity Phosphatase Regulation in Neurons and Glial Cells
- PMID: 31018603
- PMCID: PMC6514851
- DOI: 10.3390/ijms20081999
Dual-Specificity Phosphatase Regulation in Neurons and Glial Cells
Abstract
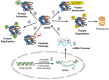
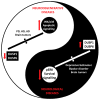
Dual-specificity protein phosphatases comprise a protein phosphatase subfamily with selectivity towards mitogen-activated protein (MAP) kinases, also named MKPs, or mitogen-activated protein kinase (MAPK) phosphatases. As powerful regulators of the intensity and duration of MAPK signaling, a relevant role is envisioned for dual-specificity protein phosphatases (DUSPs) in the regulation of biological processes in the nervous system, such as differentiation, synaptic plasticity, and survival. Important neural mediators include nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) that contribute to DUSP transcriptional induction and post-translational mechanisms of DUSP protein stabilization to maintain neuronal survival and differentiation. Potent DUSP gene inducers also include cannabinoids, which preserve DUSP activity in inflammatory conditions. Additionally, nucleotides activating P2X7 and P2Y13 nucleotide receptors behave as novel players in the regulation of DUSP function. They increase cell survival in stressful conditions, regulating DUSP protein turnover and inducing DUSP gene expression. In general terms, in the context of neural cells exposed to damaging conditions, the recovery of DUSP activity is neuroprotective and counteracts pro-apoptotic over-activation of p38 and JNK. In addition, remarkable changes in DUSP function take place during the onset of neuropathologies. The restoration of proper DUSP levels and recovery of MAPK homeostasis underlie the therapeutic effect, indicating that DUSPs can be relevant targets for brain diseases.
Keywords: BDNF; MAP kinases; P2X7; P2Y13; astrocytes; cannabinoids, granule neurons; dual-specificity phosphatases; nucleotide receptors.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


Similar articles
-
Spatiotemporal regulation of ERK2 by dual specificity phosphatases.J Biol Chem. 2008 Sep 26;283(39):26612-23. doi: 10.1074/jbc.M801500200. Epub 2008 Jul 23. J Biol Chem. 2008. PMID: 18650424 Free PMC article.
-
Dual-specificity phosphatases: critical regulators with diverse cellular targets.Biochem J. 2009 Mar 15;418(3):475-89. doi: 10.1042/bj20082234. Biochem J. 2009. PMID: 19228121 Review.
-
Dual-Specificity Phosphatases in Immunity and Infection: An Update.Int J Mol Sci. 2019 Jun 2;20(11):2710. doi: 10.3390/ijms20112710. Int J Mol Sci. 2019. PMID: 31159473 Free PMC article. Review.
-
Comparative analysis of dual specificity protein phosphatase genes 1, 2 and 5 in response to immune challenges in Japanese flounder Paralichthys olivaceus.Fish Shellfish Immunol. 2017 Sep;68:368-376. doi: 10.1016/j.fsi.2017.07.042. Epub 2017 Jul 22. Fish Shellfish Immunol. 2017. PMID: 28743632
-
P2X7 Nucleotide and EGF Receptors Exert Dual Modulation of the Dual-Specificity Phosphatase 6 (MKP-3) in Granule Neurons and Astrocytes, Contributing to Negative Feedback on ERK Signaling.Front Mol Neurosci. 2018 Jan 10;10:448. doi: 10.3389/fnmol.2017.00448. eCollection 2017. Front Mol Neurosci. 2018. PMID: 29375309 Free PMC article.
Cited by
-
Developmental dynamics of the single nucleus regulatory landscape of pig hippocampus.Sci China Life Sci. 2023 Nov;66(11):2614-2628. doi: 10.1007/s11427-022-2345-2. Epub 2023 Jul 6. Sci China Life Sci. 2023. PMID: 37428306
-
Dual-Specificity Phosphatase 15 (DUSP15) Modulates Notch Signaling by Enhancing the Stability of Notch Protein.Mol Neurobiol. 2021 May;58(5):2204-2214. doi: 10.1007/s12035-020-02254-0. Epub 2021 Jan 8. Mol Neurobiol. 2021. PMID: 33417224
-
Multifactorial etiology of progressive supranuclear palsy (PSP): the genetic component.Acta Neuropathol. 2025 Jun 4;149(1):58. doi: 10.1007/s00401-025-02898-z. Acta Neuropathol. 2025. PMID: 40465013 Free PMC article. Review.
-
High-quality genome assemblies provide clues on the evolutionary advantage of blue peafowl over green peafowl.Heliyon. 2023 Jul 22;9(8):e18571. doi: 10.1016/j.heliyon.2023.e18571. eCollection 2023 Aug. Heliyon. 2023. PMID: 37576271 Free PMC article.
-
The MAPK/ERK Pathway and the Role of DUSP1 in JCPyV Infection of Primary Astrocytes.Viruses. 2021 Sep 14;13(9):1834. doi: 10.3390/v13091834. Viruses. 2021. PMID: 34578413 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

