Analysis of Surface Levels of IL-1 Receptors and Macrophage Scavenger Receptor I in Peripheral Immune Cells of Patients With Alzheimer Disease
- PMID: 31018751
- PMCID: PMC6552296
- DOI: 10.1177/0891988719841728
Analysis of Surface Levels of IL-1 Receptors and Macrophage Scavenger Receptor I in Peripheral Immune Cells of Patients With Alzheimer Disease
Abstract
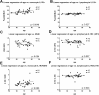
Increased concentrations of interleukin 1 (IL-1) in the cerebrospinal fluid and serum of patients with Alzheimer disease (AD) reduced phagocytic capacity point to an inflammatory activation of mononuclear phagocytes in AD. Interleukin 1 receptors (IL-1R) and the macrophage scavenger receptor I (MSRI) are important players in IL-1 signaling and phagocytosis. In 20 patients with AD and 17 controls, IL-1RI, IL-1RII, and MSRI were assessed on peripheral blood mononuclear cells by flow cytometry. IL-1β, soluble IL-1 receptors, and IL-1R antagonist (IL-1Ra) were measured by enzyme-linked immunosorbent assay. The fraction of IL-1RI+ monocytes was increased by 10% and the expression of MSRI was reduced by 12% in AD. A 3.6% increased fraction of IL-1RI+ lymphocytes was accompanied by a 6.1% reduced expression of IL-1RII. The IL-1RI on monocytes and lymphocytes discriminated patients with AD with an accuracy of 0.79 and 0.75, respectively. The IL-1Ra was elevated in AD. Changes in the expression of IL-1 receptors and MSRI on peripheral blood cells fit to the concept of a proinflammatory state of the peripheral immune system. However, the observed differences are not strong enough to suggest their application as biomarkers for AD.
Keywords: Alzheimer disease; IL-1RI; MSRI; biomarker; dementia; neurodegeneration; neuroinflammation.
Conflict of interest statement
Figures



References
-
- Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci. 2015;16(6):358–372. - PubMed
-
- Simard AR, Soulet D, Gowing G, Julien JP, Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron. 2006;49(4):489–502. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

