Case report: reinitiating pembrolizumab treatment after small bowel perforation
- PMID: 31018834
- PMCID: PMC6482547
- DOI: 10.1186/s12885-019-5577-5
Case report: reinitiating pembrolizumab treatment after small bowel perforation
Abstract
Background: Immune checkpoint inhibitors (ICIs) have emerged as paradigm shifting treatment options for a number of cancers. Six antibodies targeting the immune checkpoint proteins programmed cell death 1 (PD-1), programmed cell death 1 ligand 1 (PD-L1) or cytotoxic T-lymphocyte associated protein 4 (CTLA4) have been approved. In some cases, response rates have been impressive, but not uniformly so and not consistently; similarly, toxicity to this class of therapeutic is often unpredictable and can be life threatening. Predicting treatment response and toxicity are two main obstacles to truly individualize treatment with ICIs. One of the most severe and life-threatening adverse events is colitis induced colonic perforation, estimated to occur in 1.0 to 1.5% of patients treated with ICIs. An important question to address is, under what circumstances is it appropriate to reinitiate ICI treatment post-bowel perforation?
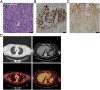
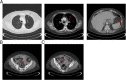
Case presentation: The patient is a 62-year-old woman, who presented with stage IV lung cancer. Immunohistochemical staining indicated that 80% of the patient's tumor cells expressed PD-L1. The patient was started on a three-week cycle of pembrolizumab. Subsequent reducing in tumor burden was observed within ten weeks. Initially, pembrolizumab was tolerated fairly well, with the exception of immunotherapy related hypothyroidism. However, the patient experienced a second, more serious immune-related adverse event (irAE), in the form of enteritis, which led to small bowel perforation and necessitated exploratory laparotomy. The concerning part of the small bowel was resected, and a primary anastomosis was created. Based on the pathological and surgical findings, the patient was diagnosed with pembrolizumab-associated small bowel perforation. The patient recovered well from surgery and, considering the patient's remarkable response to treatment, a collective decision was made to reinitiate pembrolizumab on post-operative day twenty-eight. The patient is continuing her immunotherapy with ongoing partial response and is able to continue her full-time job.
Conclusions: This case report highlights the challenges of identifying patients likely to respond to ICIs and those that are likely to experience irAEs and it discusses the impressive work that has been done to start to address these challenges. Lastly, the topic of reinitiating pembrolizumab treatment even after colonic perforation is discussed.
Keywords: Bowel perforation; CTLA4; Cancer; Immune checkpoint inhibitors; Immune-related adverse events; Immunotherapy; PD-1; PD-L1; Pembrolizumab; Toxicity.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
The patient provided written informed consent to publish this report and associated images.
Competing interests
Y.B. has served on advisory boards of Astra Zeneca, Abbvie, and Caris Life Sciences. The authors have no other conflicts to disclose.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


Similar articles
-
Peripheral Blood Markers Identify Risk of Immune-Related Toxicity in Advanced Non-Small Cell Lung Cancer Treated with Immune-Checkpoint Inhibitors.Oncologist. 2019 Aug;24(8):1128-1136. doi: 10.1634/theoncologist.2018-0563. Epub 2019 Apr 23. Oncologist. 2019. PMID: 31015312 Free PMC article.
-
Actualités et perspectives concernant l’immunothérapie en oncologie thoracique.Bull Cancer. 2018 Dec;105 Suppl 1:S16-S23. doi: 10.1016/S0007-4551(18)30386-2. Bull Cancer. 2018. PMID: 30595194 Review. French.
-
New insight in endocrine-related adverse events associated to immune checkpoint blockade.Best Pract Res Clin Endocrinol Metab. 2020 Jan;34(1):101370. doi: 10.1016/j.beem.2019.101370. Epub 2019 Dec 11. Best Pract Res Clin Endocrinol Metab. 2020. PMID: 31983543 Review.
-
Clinical outcomes in non-small cell lung cancer patients with an ultra-high expression of programmed death ligand-1 treated using pembrolizumab as a first-line therapy: A retrospective multicenter cohort study in Japan.PLoS One. 2019 Jul 31;14(7):e0220570. doi: 10.1371/journal.pone.0220570. eCollection 2019. PLoS One. 2019. PMID: 31365588 Free PMC article.
-
Isolated neutropenia as a rare but serious adverse event secondary to immune checkpoint inhibition.J Immunother Cancer. 2019 Jul 5;7(1):169. doi: 10.1186/s40425-019-0648-3. J Immunother Cancer. 2019. PMID: 31277704 Free PMC article.
Cited by
-
Immune-related adverse events-pembrolizumab-induced colitis-the importance of early diagnosis and treatment: A case report and review of the literature.Int J Immunopathol Pharmacol. 2025 Jan-Dec;39:3946320251326699. doi: 10.1177/03946320251326699. Epub 2025 Apr 15. Int J Immunopathol Pharmacol. 2025. PMID: 40231646 Free PMC article. Review.
-
Endoscopic insights into digestive-related adverse effects of immune checkpoint inhibitors: A narrative review.World J Gastrointest Endosc. 2025 Jul 16;17(7):107798. doi: 10.4253/wjge.v17.i7.107798. World J Gastrointest Endosc. 2025. PMID: 40677571 Free PMC article. Review.
-
A real-world drug safety surveillance study from the FAERS database of hepatocellular carcinoma patients receiving pembrolizumab alone and plus lenvatinib.Sci Rep. 2025 Jan 9;15(1):1425. doi: 10.1038/s41598-025-85831-4. Sci Rep. 2025. PMID: 39789316 Free PMC article.
-
Differential diagnosis and management of immune checkpoint inhibitor-induced colitis: A comprehensive review.World J Exp Med. 2021 Dec 30;11(6):79-92. doi: 10.5493/wjem.v11.i6.79. eCollection 2021 Dec 30. World J Exp Med. 2021. PMID: 36246150 Free PMC article. Review.
-
Immune Checkpoint Inhibitor-Associated Colitis: From Mechanism to Management.Front Immunol. 2021 Dec 21;12:800879. doi: 10.3389/fimmu.2021.800879. eCollection 2021. Front Immunol. 2021. PMID: 34992611 Free PMC article. Review.
References
-
- Thomas R, Fysh ETH, Smith NA, Lee P, Kwan BCH, Yap E, Horwood FC, Piccolo F, Lam DCL, Garske LA, et al. Effect of an indwelling pleural catheter vs talc Pleurodesis on hospitalization days in patients with malignant pleural effusion: the AMPLE randomized clinical trial. JAMA. 2017;318(19):1903–1912. doi: 10.1001/jama.2017.17426. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

