Tmem178 negatively regulates store-operated calcium entry in myeloid cells via association with STIM1
- PMID: 31018906
- PMCID: PMC7102427
- DOI: 10.1016/j.jaut.2019.04.015
Tmem178 negatively regulates store-operated calcium entry in myeloid cells via association with STIM1
Abstract
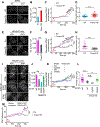
Store-operated calcium entry (SOCE) modulates cytosolic calcium in multiple cells. Endoplasmic reticulum (ER)-localized STIM1 and plasma membrane (PM)-localized ORAI1 are two main components of SOCE. STIM1:ORAI1 association requires STIM1 oligomerization, its re-distribution to ER-PM junctions, and puncta formation. However, little is known about the negative regulation of these steps to prevent calcium overload. Here, we identified Tmem178 as a negative modulator of STIM1 puncta formation in myeloid cells. Using site-directed mutagenesis, co-immunoprecipitation assays and FRET imaging, we determined that Tmem178:STIM1 association occurs via their transmembrane motifs. Mutants that increase Tmem178:STIM1 association reduce STIM1 puncta formation, SOCE activation, impair inflammatory cytokine production in macrophages and osteoclastogenesis. Mutants that reduce Tmem178:STIM1 association reverse these effects. Furthermore, exposure to plasma from arthritic patients decreases Tmem178 expression, enhances SOCE activation and cytoplasmic calcium. In conclusion, Tmem178 modulates the rate-limiting step of STIM1 puncta formation and therefore controls SOCE in inflammatory conditions.
Keywords: Macrophage activation; Osteoclastogenesis; SOCE; STIM1; Tmem178.
Copyright © 2019. Published by Elsevier Ltd.
Conflict of interest statement
Declare of interests
The authors declare that they have no conflicts of interest with the contents of this article.
Figures





References
-
- Berridge MJ, Bootman MD, Roderick HL, Calcium signalling: dynamics, homeostasis and remodelling, Nat. Rev. Mol. Cell Biol 4 (2003) 517–529. - PubMed
-
- Berridge MJ, The inositol trisphosphate/calcium signaling pathway in health and disease, Physiol. Rev 96 (2016) 1261–1296. - PubMed
-
- Feske S, Calcium signalling in lymphocyte activation and disease, Nat. Rev. Immunol 7 (2007) 690–702. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

