Trapping the Enemy: Vermamoeba vermiformis Circumvents Faustovirus Mariensis Dissemination by Enclosing Viral Progeny inside Cysts
- PMID: 31019058
- PMCID: PMC6600206
- DOI: 10.1128/JVI.00312-19
Trapping the Enemy: Vermamoeba vermiformis Circumvents Faustovirus Mariensis Dissemination by Enclosing Viral Progeny inside Cysts
Abstract
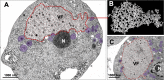
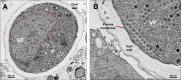
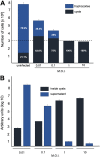
Viruses depend on cells to replicate and can cause considerable damage to their hosts. However, hosts have developed a plethora of antiviral mechanisms to counterattack or prevent viral replication and to maintain homeostasis. Advantageous features are constantly being selected, affecting host-virus interactions and constituting a harsh race for supremacy in nature. Here, we describe a new antiviral mechanism unveiled by the interaction between a giant virus and its amoebal host. Faustovirus mariensis infects Vermamoeba vermiformis, a free-living amoeba, and induces cell lysis to disseminate into the environment. Once infected, the cells release a soluble factor that triggers the encystment of neighbor cells, preventing their infection. Remarkably, infected cells stimulated by the factor encyst and trap the viruses and viral factories inside cyst walls, which are no longer viable and cannot excyst. This unprecedented mechanism illustrates that a plethora of antiviral strategies remains to be discovered in nature.IMPORTANCE Understanding how viruses of microbes interact with its hosts is not only important from a basic scientific point of view but also for a better comprehension of the evolution of life. Studies involving large and giant viruses have revealed original and outstanding mechanisms concerning virus-host relationships. Here, we report a mechanism developed by Vermamoeba vermiformis, a free-living amoeba, to reduce Faustovirus mariensis dissemination. Once infected, V. vermiformis cells release a factor that induces the encystment of neighbor cells, preventing infection of further cells and/or trapping the viruses and viral factories inside the cyst walls. This phenomenon reinforces the need for more studies regarding large/giant viruses and their hosts.
Keywords: antiviral; cysts; faustovirus; vermoameba; virus control.
Copyright © 2019 American Society for Microbiology.
Figures







Similar articles
-
Giant virus vs amoeba: fight for supremacy.Virol J. 2019 Nov 4;16(1):126. doi: 10.1186/s12985-019-1244-3. Virol J. 2019. PMID: 31684962 Free PMC article. Review.
-
In-depth analysis of the replication cycle of Orpheovirus.Virol J. 2019 Dec 16;16(1):158. doi: 10.1186/s12985-019-1268-8. Virol J. 2019. PMID: 31842897 Free PMC article.
-
Diversity of Giant Viruses Infecting Vermamoeba vermiformis.Front Microbiol. 2022 Apr 22;13:808499. doi: 10.3389/fmicb.2022.808499. eCollection 2022. Front Microbiol. 2022. PMID: 35602053 Free PMC article. Review.
-
A Rapid Strategy for the Isolation of New Faustoviruses from Environmental Samples Using Vermamoeba vermiformis.J Vis Exp. 2016 Jun 4;(112):54104. doi: 10.3791/54104. J Vis Exp. 2016. PMID: 27341059 Free PMC article.
-
Faustovirus-Like Asfarvirus in Hematophagous Biting Midges and Their Vertebrate Hosts.Front Microbiol. 2015 Dec 16;6:1406. doi: 10.3389/fmicb.2015.01406. eCollection 2015. Front Microbiol. 2015. PMID: 26733117 Free PMC article.
Cited by
-
A history of over 40 years of potentially pathogenic free-living amoeba studies in Brazil - a systematic review.Mem Inst Oswaldo Cruz. 2022 Jul 1;117:e210373. doi: 10.1590/0074-02760210373. eCollection 2022. Mem Inst Oswaldo Cruz. 2022. PMID: 35792751 Free PMC article.
-
Giant virus vs amoeba: fight for supremacy.Virol J. 2019 Nov 4;16(1):126. doi: 10.1186/s12985-019-1244-3. Virol J. 2019. PMID: 31684962 Free PMC article. Review.
-
In-depth analysis of the replication cycle of Orpheovirus.Virol J. 2019 Dec 16;16(1):158. doi: 10.1186/s12985-019-1268-8. Virol J. 2019. PMID: 31842897 Free PMC article.
-
Diversity of Giant Viruses Infecting Vermamoeba vermiformis.Front Microbiol. 2022 Apr 22;13:808499. doi: 10.3389/fmicb.2022.808499. eCollection 2022. Front Microbiol. 2022. PMID: 35602053 Free PMC article. Review.
-
Asfarviruses and Closely Related Giant Viruses.Viruses. 2023 Apr 20;15(4):1015. doi: 10.3390/v15041015. Viruses. 2023. PMID: 37112995 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

