Telomere DNA G-quadruplex folding within actively extending human telomerase
- PMID: 31019071
- PMCID: PMC6510993
- DOI: 10.1073/pnas.1814777116
Telomere DNA G-quadruplex folding within actively extending human telomerase
Abstract
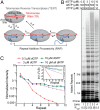
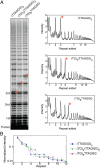
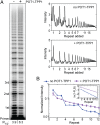
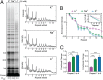
Telomerase reverse transcribes short guanine (G)-rich DNA repeat sequences from its internal RNA template to maintain telomere length. G-rich telomere DNA repeats readily fold into G-quadruplex (GQ) structures in vitro, and the presence of GQ-prone sequences throughout the genome introduces challenges to replication in vivo. Using a combination of ensemble and single-molecule telomerase assays, we discovered that GQ folding of the nascent DNA product during processive addition of multiple telomere repeats modulates the kinetics of telomerase catalysis and dissociation. Telomerase reactions performed with telomere DNA primers of varying sequence or using GQ-stabilizing K+ versus GQ-destabilizing Li+ salts yielded changes in DNA product profiles consistent with formation of GQ structures within the telomerase-DNA complex. Addition of the telomerase processivity factor POT1-TPP1 altered the DNA product profile, but was not sufficient to recover full activity in the presence of Li+ cations. This result suggests GQ folding synergizes with POT1-TPP1 to support telomerase function. Single-molecule Förster resonance energy transfer experiments reveal complex DNA structural dynamics during real-time catalysis in the presence of K+ but not Li+, supporting the notion of nascent product folding within the active telomerase complex. To explain the observed distributions of telomere products, we globally fit telomerase time-series data to a kinetic model that converges to a set of rate constants describing each successive telomere repeat addition cycle. Our results highlight the potential influence of the intrinsic folding properties of telomere DNA during telomerase catalysis, and provide a detailed characterization of GQ modulation of polymerase function.
Keywords: DNA structure; G quadruplex; POT1–TPP1; telomerase; telomere.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
POT1-TPP1 Binding and Unfolding of Telomere DNA Discriminates against Structural Polymorphism.J Mol Biol. 2016 Jul 3;428(13):2695-708. doi: 10.1016/j.jmb.2016.04.031. Epub 2016 May 10. J Mol Biol. 2016. PMID: 27173378 Free PMC article.
-
G-quadruplex formation in telomeres enhances POT1/TPP1 protection against RPA binding.Proc Natl Acad Sci U S A. 2014 Feb 25;111(8):2990-5. doi: 10.1073/pnas.1321436111. Epub 2014 Feb 10. Proc Natl Acad Sci U S A. 2014. PMID: 24516170 Free PMC article.
-
POT1-TPP1 enhances telomerase processivity by slowing primer dissociation and aiding translocation.EMBO J. 2010 Mar 3;29(5):924-33. doi: 10.1038/emboj.2009.409. Epub 2010 Jan 21. EMBO J. 2010. PMID: 20094033 Free PMC article.
-
POT1-TPP1 telomere length regulation and disease.Comput Struct Biotechnol J. 2020 Jul 3;18:1939-1946. doi: 10.1016/j.csbj.2020.06.040. eCollection 2020. Comput Struct Biotechnol J. 2020. PMID: 32774788 Free PMC article. Review.
-
Folding of guanine quadruplex molecules-funnel-like mechanism or kinetic partitioning? An overview from MD simulation studies.Biochim Biophys Acta Gen Subj. 2017 May;1861(5 Pt B):1246-1263. doi: 10.1016/j.bbagen.2016.12.008. Epub 2016 Dec 13. Biochim Biophys Acta Gen Subj. 2017. PMID: 27979677 Review.
Cited by
-
Structure of active human telomerase with telomere shelterin protein TPP1.Nature. 2022 Apr;604(7906):578-583. doi: 10.1038/s41586-022-04582-8. Epub 2022 Apr 13. Nature. 2022. PMID: 35418675 Free PMC article.
-
G-quadruplexes as pivotal components of cis-regulatory elements in the human genome.BMC Biol. 2024 Aug 26;22(1):177. doi: 10.1186/s12915-024-01971-5. BMC Biol. 2024. PMID: 39183303 Free PMC article.
-
Streamlining effects of extra telomeric repeat on telomeric DNA folding revealed by fluorescence-force spectroscopy.Nucleic Acids Res. 2019 Dec 2;47(21):11044-11056. doi: 10.1093/nar/gkz906. Nucleic Acids Res. 2019. PMID: 31617570 Free PMC article.
-
The Processivity of Telomerase: Insights from Kinetic Simulations and Analyses.Molecules. 2021 Dec 13;26(24):7532. doi: 10.3390/molecules26247532. Molecules. 2021. PMID: 34946615 Free PMC article.
-
Pot1b -/- tumors activate G-quadruplex-induced DNA damage to promote telomere hyper-elongation.Nucleic Acids Res. 2023 Sep 22;51(17):9227-9247. doi: 10.1093/nar/gkad648. Nucleic Acids Res. 2023. PMID: 37560909 Free PMC article.
References
-
- de Lange T. Shelterin: The protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110. - PubMed
-
- de Lange T. How shelterin solves the telomere end-protection problem. Cold Spring Harb Symp Quant Biol. 2010;75:167–177. - PubMed
-
- Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42:301–334. - PubMed

