Genome-wide CRISPR screen for Zika virus resistance in human neural cells
- PMID: 31019072
- PMCID: PMC6510995
- DOI: 10.1073/pnas.1900867116
Genome-wide CRISPR screen for Zika virus resistance in human neural cells
Abstract
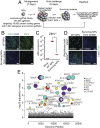
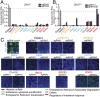
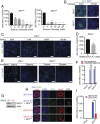
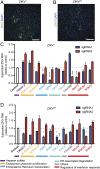
Zika virus (ZIKV) is a neurotropic and neurovirulent arbovirus that has severe detrimental impact on the developing human fetal brain. To date, little is known about the factors required for ZIKV infection of human neural cells. We identified ZIKV host genes in human pluripotent stem cell (hPSC)-derived neural progenitors (NPs) using a genome-wide CRISPR-Cas9 knockout screen. Mutations of host factors involved in heparan sulfation, endocytosis, endoplasmic reticulum processing, Golgi function, and interferon activity conferred resistance to infection with the Uganda strain of ZIKV and a more recent North American isolate. Host genes essential for ZIKV replication identified in human NPs also provided a low level of protection against ZIKV in isogenic human astrocytes. Our findings provide insights into host-dependent mechanisms for ZIKV infection in the highly vulnerable human NP cells and identify molecular targets for potential therapeutic intervention.
Keywords: CRISPR screen; Zika virus; fetal CNS infection; human pluripotent stem cells; neural progenitors.
Conflict of interest statement
Conflict of interest statement: R.J. is a cofounder of Fate Therapeutics, Fulcrum Therapeutics and Omega Therapeutics. I.B. and L.G. are cofounders of E25Bio, Inc.
Figures

Similar articles
-
A CRISPR Activation Screen Identifies Genes That Protect against Zika Virus Infection.J Virol. 2019 Jul 30;93(16):e00211-19. doi: 10.1128/JVI.00211-19. Print 2019 Aug 15. J Virol. 2019. PMID: 31142663 Free PMC article.
-
Zika Virus Infection Induces DNA Damage Response in Human Neural Progenitors That Enhances Viral Replication.J Virol. 2019 Sep 30;93(20):e00638-19. doi: 10.1128/JVI.00638-19. Print 2019 Oct 15. J Virol. 2019. PMID: 31375586 Free PMC article.
-
Human induced pluripotent stem cell-derived glial cells and neural progenitors display divergent responses to Zika and dengue infections.Proc Natl Acad Sci U S A. 2018 Jul 3;115(27):7117-7122. doi: 10.1073/pnas.1719266115. Epub 2018 Jun 18. Proc Natl Acad Sci U S A. 2018. PMID: 29915057 Free PMC article.
-
The impact of Zika virus in the brain.Biochem Biophys Res Commun. 2017 Oct 28;492(4):603-607. doi: 10.1016/j.bbrc.2017.01.074. Epub 2017 Jan 17. Biochem Biophys Res Commun. 2017. PMID: 28108286 Review.
-
Zika infection and the development of neurological defects.Cell Microbiol. 2017 Jun;19(6). doi: 10.1111/cmi.12744. Epub 2017 May 3. Cell Microbiol. 2017. PMID: 28370966 Review.
Cited by
-
Crippling life support for SARS-CoV-2 and other viruses through synthetic lethality.J Cell Biol. 2020 Oct 5;219(10):e202006159. doi: 10.1083/jcb.202006159. J Cell Biol. 2020. PMID: 32785687 Free PMC article.
-
Deciphering flavivirus-host interactions using quantitative proteomics.Curr Opin Immunol. 2020 Oct;66:90-97. doi: 10.1016/j.coi.2020.06.002. Epub 2020 Jul 15. Curr Opin Immunol. 2020. PMID: 32682290 Free PMC article. Review.
-
CRISPR-Cas system to discover host-virus interactions in Flaviviridae.Virol J. 2023 Oct 27;20(1):247. doi: 10.1186/s12985-023-02216-7. Virol J. 2023. PMID: 37891676 Free PMC article. Review.
-
Studying Human Neurodevelopment and Diseases Using 3D Brain Organoids.J Neurosci. 2020 Feb 5;40(6):1186-1193. doi: 10.1523/JNEUROSCI.0519-19.2019. J Neurosci. 2020. PMID: 32024767 Free PMC article. Review.
-
Genome Editing in iPSC-Based Neural Systems: From Disease Models to Future Therapeutic Strategies.Front Genome Ed. 2021 Mar 15;3:630600. doi: 10.3389/fgeed.2021.630600. eCollection 2021. Front Genome Ed. 2021. PMID: 34713254 Free PMC article. Review.
References
-
- Li H, Saucedo-Cuevas L, Shresta S, Gleeson JG. The neurobiology of Zika virus. Neuron. 2016;92:949–958. - PubMed
-
- Martines RB, et al. Pathology of congenital Zika syndrome in Brazil: A case series. Lancet. 2016;388:898–904. - PubMed
-
- Mlakar J, et al. Zika virus associated with microcephaly. N Engl J Med. 2016;374:951–958. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

