Nanoscale imaging reveals miRNA-mediated control of functional states of dendritic spines
- PMID: 31019087
- PMCID: PMC6511049
- DOI: 10.1073/pnas.1819374116
Nanoscale imaging reveals miRNA-mediated control of functional states of dendritic spines
Abstract
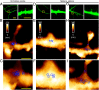
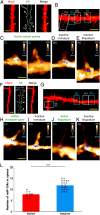
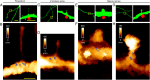
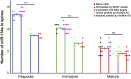
Dendritic spines are major loci of excitatory inputs and undergo activity-dependent structural changes that contribute to synaptic plasticity and memory formation. Despite the existence of various classification types of spines, how they arise and which molecular components trigger their structural plasticity remain elusive. microRNAs (miRNAs) have emerged as critical regulators of synapse development and plasticity via their control of gene expression. Brain-specific miR-134s likely regulate the morphological maturation of spines, but their subcellular distributions and functional impacts have rarely been assessed. Here, we exploited atomic force microscopy to visualize in situ miR-134s, which indicated that they are mainly distributed at nearby dendritic shafts and necks of spines. The abundance of miR-134s varied between morphologically and functionally distinct spine types, and their amounts were inversely correlated with their postulated maturation stages. Moreover, spines exhibited reduced contents of miR-134s when selectively stimulated with beads containing brain-derived neurotropic factor (BDNF). Taken together, in situ visualizations of miRNAs provided unprecedented insights into the "inverse synaptic-tagging" roles of miR-134s that are selective to inactive/irrelevant synapses and potentially a molecular means for modifying synaptic connectivity via structural alteration.
Keywords: atomic force microscopy; dendritic spines; force mapping; microRNAs; structural plasticity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Hering H, Sheng M. Dendritic spines: Structure, dynamics and regulation. Nat Rev Neurosci. 2001;2:880–888. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

