Nusinersen in later-onset spinal muscular atrophy: Long-term results from the phase 1/2 studies
- PMID: 31019106
- PMCID: PMC6541434
- DOI: 10.1212/WNL.0000000000007527
Nusinersen in later-onset spinal muscular atrophy: Long-term results from the phase 1/2 studies
Abstract
Objective: To report results of intrathecal nusinersen in children with later-onset spinal muscular atrophy (SMA).
Methods: Analyses included children from a phase 1b/2a study (ISIS-396443-CS2; NCT01703988) who first received nusinersen during that study and were eligible to continue treatment in the extension study (ISIS-396443-CS12; NCT02052791). The phase 1b/2a study was a 253-day, ascending dose (3, 6, 9, 12 mg), multiple-dose, open-label, multicenter study that enrolled children with SMA aged 2-15 years. The extension study was a 715-day, single-dose level (12 mg) study. Time between studies varied by participant (196-413 days). Assessments included the Hammersmith Functional Motor Scale-Expanded (HFMSE), Upper Limb Module (ULM), 6-Minute Walk Test (6MWT), compound muscle action potential (CMAP), and quantitative multipoint incremental motor unit number estimation. Safety also was assessed.
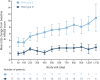
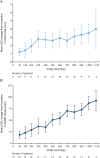
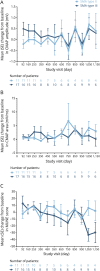
Results: Twenty-eight children were included (SMA type II, n = 11; SMA type III, n = 17). Mean HFMSE scores, ULM scores, and 6MWT distances improved by the day 1,150 visit (HFMSE: SMA type II, +10.8 points; SMA type III, +1.8 points; ULM: SMA type II, +4.0 points; 6MWT: SMA type III, +92.0 meters). Mean CMAP values remained relatively stable. No children discontinued treatment due to adverse events.
Conclusions: Nusinersen treatment over ∼3 years resulted in motor function improvements and disease activity stabilization not observed in natural history cohorts. These results document the long-term benefit of nusinersen in later-onset SMA, including SMA type III.
Clinicaltrialsgov identifier: NCT01703988 (ISIS-396443-CS2); NCT02052791 (ISIS-396443-CS12).
Classification of evidence: This study provides Class IV evidence that nusinersen improves motor function in children with later-onset SMA.
Copyright © 2019 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures
Comment in
-
Nusinersen for spinal muscular atrophy: Not just for babies?Neurology. 2019 May 21;92(21):985-986. doi: 10.1212/WNL.0000000000007559. Epub 2019 Apr 24. Neurology. 2019. PMID: 31019098 No abstract available.
Similar articles
-
Safety and Treatment Effects of Nusinersen in Longstanding Adult 5q-SMA Type 3 - A Prospective Observational Study.J Neuromuscul Dis. 2019;6(4):453-465. doi: 10.3233/JND-190416. J Neuromuscul Dis. 2019. PMID: 31594243 Free PMC article.
-
Nusinersen for adults with spinal muscular atrophy.Neurol Sci. 2023 Jul;44(7):2393-2400. doi: 10.1007/s10072-023-06698-9. Epub 2023 Mar 1. Neurol Sci. 2023. PMID: 36854931
-
Nusinersen versus Sham Control in Later-Onset Spinal Muscular Atrophy.N Engl J Med. 2018 Feb 15;378(7):625-635. doi: 10.1056/NEJMoa1710504. N Engl J Med. 2018. PMID: 29443664 Clinical Trial.
-
Drug treatment for spinal muscular atrophy types II and III.Cochrane Database Syst Rev. 2020 Jan 6;1(1):CD006282. doi: 10.1002/14651858.CD006282.pub5. Cochrane Database Syst Rev. 2020. PMID: 32006461 Free PMC article.
-
Motor Function and Safety of Nusinersen and Risdiplam in Asian Patients with Types 2-4 Spinal Muscular Atrophy (SMA): A Systematic Review and Meta-Analysis.Adv Ther. 2025 Apr;42(4):1611-1626. doi: 10.1007/s12325-024-03101-7. Epub 2025 Feb 17. Adv Ther. 2025. PMID: 39960588
Cited by
-
Spinal muscular atrophy - insights and challenges in the treatment era.Nat Rev Neurol. 2020 Dec;16(12):706-715. doi: 10.1038/s41582-020-00413-4. Epub 2020 Oct 14. Nat Rev Neurol. 2020. PMID: 33057172 Review.
-
Survival motor neuron deficiency slows myoblast fusion through reduced myomaker and myomixer expression.J Cachexia Sarcopenia Muscle. 2021 Aug;12(4):1098-1116. doi: 10.1002/jcsm.12740. Epub 2021 Jun 11. J Cachexia Sarcopenia Muscle. 2021. PMID: 34115448 Free PMC article.
-
Antisense modulation of IL7R splicing to control sIL7R expression in human CD4+ T cells.RNA. 2022 Aug;28(8):1058-1073. doi: 10.1261/rna.079137.122. Epub 2022 May 25. RNA. 2022. PMID: 35613883 Free PMC article.
-
Alberta Spinal Muscular Atrophy Newborn Screening-Results from Year 1 Pilot Project.Int J Neonatal Screen. 2023 Jul 27;9(3):42. doi: 10.3390/ijns9030042. Int J Neonatal Screen. 2023. PMID: 37606479 Free PMC article.
-
Effects of nusinersen after one year of treatment in 123 children with SMA type 1 or 2: a French real-life observational study.Orphanet J Rare Dis. 2020 Jun 12;15(1):148. doi: 10.1186/s13023-020-01414-8. Orphanet J Rare Dis. 2020. PMID: 32532349 Free PMC article.
References
-
- Lunn MR, Wang CH. Spinal muscular atrophy. Lancet 2008;371:2120–2133. - PubMed
-
- Darras BT, Markowitz JA, Monani UR, De Vivo DC. Spinal muscular atrophies. In: Darras BT, Royden Jones H Jr, Ryan MM, De Vivo DC, eds. Neuromuscular Disorders of Infancy, Childhood, and Adolescence: A Clinician's Approach, 2nd ed. San Diego: Academic Press; 2015:117–145.
-
- Finkel RS, Mercuri E, Darras BT, et al. . Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N Engl J Med 2017;377:1723–1732. - PubMed
-
- Finkel RS, Chiriboga CA, Vajsar J, et al. . Treatment of infantile-onset spinal muscular atrophy with nusinersen: a phase 2, open-label, dose-escalation study. Lancet 2016;388:3017–3026. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
