CD4+CXCR5+PD-1+ T Follicular Helper Cells Play a Pivotal Role in the Development of Rheumatoid Arthritis
- PMID: 31019190
- PMCID: PMC6498883
- DOI: 10.12659/MSM.914868
CD4+CXCR5+PD-1+ T Follicular Helper Cells Play a Pivotal Role in the Development of Rheumatoid Arthritis
Abstract
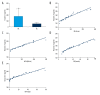
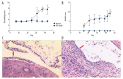
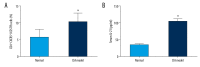
BACKGROUND T follicular helper (Tfh) cells are a subgroup of activated CD4+ T cells in the germinal centers of secondary lymphoid organs, they play critical roles in the development of many chronic autoimmune inflammatory diseases. The aim of this study was to investigate whether circulating Tfh cells contribute to the development of rheumatoid arthritis (RA). MATERIAL AND METHODS Thirty patients fulfilled the diagnosis criteria that was established by the American College of Rheumatology and 30 healthy controls were recruited. The frequency of Tfh cells in patients and collagen-induced arthritis (CIA) in DBA/1J mice were analyzed by flow cytometry. The serum IL-21 level was examined by enzyme-linked immunosorbent assay (ELISA). The mRNA expression of Blimp-1 and Bcl-6 were detected by qRT-PCR. RESULTS RA patients had more CD4⁺PD-1⁺CXCR5⁺ Tfh cells in peripheral blood compared with healthy controls, and CIA in DBA/1J mice showed similar results. Higher mRNA expression of Bcl-6 and lower Blimp-1 mRNA expression were observed in patients with RA compared to healthy controls, and the expression level of IL-21 was higher in RA patients, which was also seen in CIA mice. Furthermore, the spleen CD4⁺ICOS⁺CXCR5⁺ Tfh cells in CIA mice show significantly higher frequency than that in the control mice. The percentage of CD4⁺PD-1⁺CXCR5⁺ Tfh cells was correlated positively with the values of erythrocyte sedimentation rate (ESR) (r=0.968, P<0.001), rheumatoid factor (RF) (r=0.962, P<0.001), C-reactive protein (CRP) (r=0.953, P<0.001), and anti-cyclic citrullinated peptide antibodies (ACPA) (r=0.966, P<0.001), and the level of serum interleukin (IL)-21 in RA patients showed positive correlation with ESR (r=0.982, P<0.001), RF (r=0.959, P<0.001), CRP (r=0.951, P<0.001), and ACPA (r=0.971, P<0.001) as well. CONCLUSIONS The activated Tfh cells in the peripheral blood may be responsible for the development of RA.
Conflict of interest statement
None.
Figures


Similar articles
-
[Detection of peripheral follicular helper T cells in rheumatoid arthritis].Beijing Da Xue Xue Bao Yi Xue Ban. 2016 Dec 18;48(6):951-957. Beijing Da Xue Xue Bao Yi Xue Ban. 2016. PMID: 27987496 Chinese.
-
An imbalance between blood CD4+CXCR5+Foxp3+ Tfr cells and CD4+CXCR5+Tfh cells may contribute to the immunopathogenesis of rheumatoid arthritis.Mol Immunol. 2020 Sep;125:1-8. doi: 10.1016/j.molimm.2020.06.003. Epub 2020 Jun 28. Mol Immunol. 2020. PMID: 32610164
-
Higher frequency of peripheral blood interleukin 21 positive follicular helper T cells in patients with ankylosing spondylitis.J Rheumatol. 2013 Dec;40(12):2029-37. doi: 10.3899/jrheum.130125. Epub 2013 Nov 1. J Rheumatol. 2013. PMID: 24187103
-
Follicular helper T cells in rheumatoid arthritis.Clin Rheumatol. 2015 Sep;34(9):1489-93. doi: 10.1007/s10067-015-3028-5. Epub 2015 Jul 31. Clin Rheumatol. 2015. PMID: 26227164 Review.
-
Molecular Control of Follicular Helper T cell Development and Differentiation.Front Immunol. 2018 Oct 25;9:2470. doi: 10.3389/fimmu.2018.02470. eCollection 2018. Front Immunol. 2018. PMID: 30410493 Free PMC article. Review.
Cited by
-
Clinical effects of HBV infection on patients with Rheumatoid Arthritis and Systemic Lupus Erythematosus.Pak J Med Sci. 2023 Sep-Oct;39(5):1446-1450. doi: 10.12669/pjms.39.5.7232. Pak J Med Sci. 2023. PMID: 37680831 Free PMC article.
-
Platelets as a potential new immune coordinator in T cell-mediated aplastic anemia.Front Oncol. 2025 Jun 9;15:1568169. doi: 10.3389/fonc.2025.1568169. eCollection 2025. Front Oncol. 2025. PMID: 40552264 Free PMC article. Review.
-
Bioinformatics analysis of rheumatoid arthritis tissues identifies genes and potential drugs that are expressed specifically.Sci Rep. 2023 Mar 18;13(1):4508. doi: 10.1038/s41598-023-31438-6. Sci Rep. 2023. PMID: 36934132 Free PMC article.
-
Altered B cell compartment associated with Tfh cells in children with Henoch-Schonlein Purpura.BMC Pediatr. 2021 Sep 13;21(1):399. doi: 10.1186/s12887-021-02873-z. BMC Pediatr. 2021. PMID: 34517873 Free PMC article.
-
Human follicular helper T lymphocytes critical players in antibody responses.Einstein (Sao Paulo). 2021 Mar 5;19:eRB6077. doi: 10.31744/einstein_journal/2021RB6077. eCollection 2021. Einstein (Sao Paulo). 2021. PMID: 33681888 Free PMC article. Review.
References
-
- Smolen J, Aletaha D, Barton A, et al. Rheumatoid arthritis. Nat Rev Dis Primers. 2018;4:18001. - PubMed
-
- Scott D, Wolfe F, Huizinga T. Rheumatoid arthritis. Lancet. 2010;376(9746):1094–108. - PubMed
-
- Klareskog L, Amara K, Malmström V. Adaptive immunity in rheumatoid arthritis: Anticitrulline and other antibodies in the pathogenesis of rheumatoid arthritis. Curr Opin Rheumatol. 2014;26(1):72–79. - PubMed
-
- Agrawal S, Misra R, Aggarwal A. Autoantibodies in rheumatoid arthritis: Association with severity of disease in established RA. Clin Rheumatol. 2007;26(2):201–4. - PubMed
-
- Firestein G. Immunologic mechanisms in the pathogenesis of rheumatoid arthritis. J Clin Rheumatol. 2005;11(3 Suppl):S39–44. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

